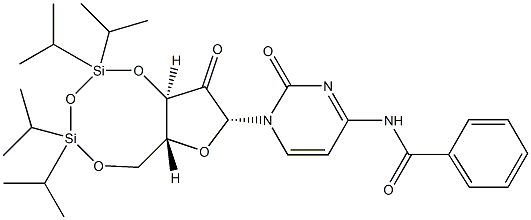
Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]- synthesis
- Product Name:Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-
- CAS Number:119411-03-9
- Molecular formula:C28H41N3O7Si2
- Molecular Weight:587.81
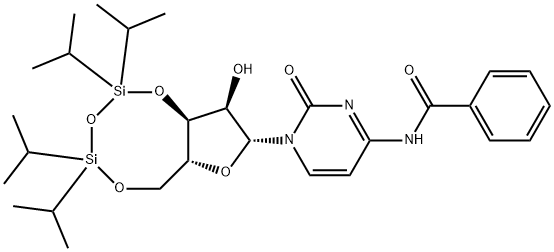
69304-43-4
47 suppliers
$45.00/250mg
![Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-](/CAS/20180808/GIF/119411-03-9.gif)
119411-03-9
7 suppliers
inquiry
Yield:119411-03-9 84%
Reaction Conditions:
Stage #1: 1-[3,5-O-(1,1,3,3-tetraisopropyldisiloxane-1,3-diyl)-β-D-ribofuranosyl]-N4-benzoylcytosinewith dimethyl sulfoxide;trifluoroacetic anhydride in tetrahydrofuran at -20; for 2 h;
Stage #2: with triethylamine in tetrahydrofuran at -15;
Steps:
15.b
Step 15b.; A mixture of the compound from step 15a (56.6 g, 96.0 mmol) and DMSO (44.3 mL, 0.624 mol) in THF (300 mL) was treated with trifluoroacetic anhydride (40.0 ml, 0.288 mol) at -20° C. for 2 hours. Triethylamine (60.2 ml, 0.432 mol) was then added slowly while keeping the internal temperature below -15° C. After addition, the cooling bath was removed and the reaction mixture was allowed to warm up to room temperature before being diluted with ethyl acetate. The organic layer was washed with H2O (three times), brine, dried (Na2SO4), filtered and evaporated. The residue was chromatographed (silica, hexanes-ethyl acetate) to give the desired compound as a yellow foam (47.6 g, 84%). ESIMS m/z=606.21 [M+H+H2O]+.
References:
US2009/274686,2009,A1 Location in patent:Page/Page column 30
93-97-0
367 suppliers
$6.00/25g
![Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-](/CAS/20180808/GIF/119411-03-9.gif)
119411-03-9
7 suppliers
inquiry

65-46-3
675 suppliers
$5.00/1g
![Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-](/CAS/20180808/GIF/119411-03-9.gif)
119411-03-9
7 suppliers
inquiry
69304-37-6
299 suppliers
$10.00/1g
![Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-](/CAS/20180808/GIF/119411-03-9.gif)
119411-03-9
7 suppliers
inquiry

13089-48-0
255 suppliers
$16.00/1g
![Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-](/CAS/20180808/GIF/119411-03-9.gif)
119411-03-9
7 suppliers
inquiry