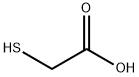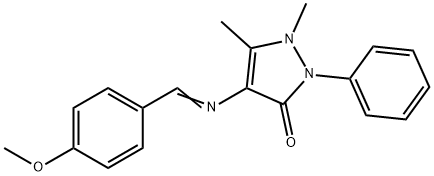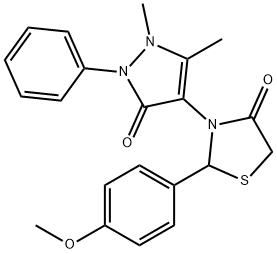
3-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-2-(4-methoxyphenyl)-1,3-thiazolidin-4-one synthesis
- Product Name:3-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-2-(4-methoxyphenyl)-1,3-thiazolidin-4-one
- CAS Number:125298-58-0
- Molecular formula:C21H21N3O3S
- Molecular Weight:395.47
Yield:125298-58-0 85%
Reaction Conditions:
in neat (no solvent) at 170;Microwave irradiation;Green chemistry;
Steps:
Microwave-Mediated Activated Fly Ash-Catalyzed General Synthesis of 1, 2-Dihydro-4-(2-(2-hydroxyphenyl)-4-oxothiazolidin-3-yl)-2, 3-dimethyl-1-phenylpyrazol-5-one 4(a):.
General procedure: A mixture of 4-(2-hydroxybenzylideneamino)-1, 2-dihydro-2, 3-dimethyl-1-phenylpyrazol-5-one (3a) (3.23 mmole, 1.0 g), SHCH2COOH (thioacetic acid) (3.23 mmole, 0.30 g), and activated fly ash (0.50 g) was taken in a 10-mL crimp-sealed, thick-walled glass tube and irradiated at 170 °C (400 W) in a commercial microwave apparatus (CEM Discover focused microwave synthesis system) in a typical solvent-free approach for 8 min. The reaction was monitored by TLC. After completion of the reaction, the pasty solid obtained was allowed to cool. To that, ethanol (15 mL) was added and stirred for 5 min, up to the dissolution of the pasty solid. The fly ash was filtered out of the mother liquor for the other reaction. The mother liquor was poured onto crushed ice and the pH of the solution was set to 10 using 10% NaHCO3 solution. The reaction mass was then stirred, filtered, and washed with cold water. The crude product was recrystallized from methanol. The same process was used for the production of other thiazolidinones 4(b-n).
References:
Shanti, Manoj Dilip;Shanti, Kaveri;Meshram, Jyotsana [Phosphorus, Sulfur and Silicon and the Related Elements,2013,vol. 188,# 10,p. 1351 - 1360]
83-07-8
358 suppliers
$5.00/100mg

125298-58-0
4 suppliers
inquiry
123-11-5
846 suppliers
$10.00/10g

125298-58-0
4 suppliers
inquiry