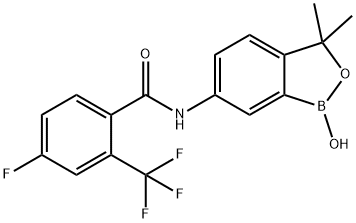
4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-(trifluoromethyl)benzamide(WXG02326) synthesis
- Product Name:4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-(trifluoromethyl)benzamide(WXG02326)
- CAS Number:1266084-51-8
- Molecular formula:C17H14BF4NO3
- Molecular Weight:367.1
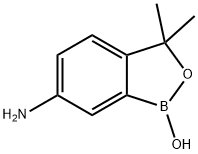
1266320-01-7
15 suppliers
inquiry
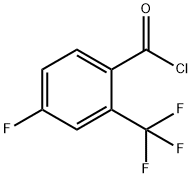
189807-21-4
102 suppliers
$10.00/1g
![4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-(trifluoromethyl)benzamide(WXG02326)](/CAS/20180629/GIF/1266084-51-8.gif)
1266084-51-8
28 suppliers
inquiry
Yield: 94%
Reaction Conditions:
Stage #1:6-amino-3,3-dimethylbenzo[c][1,2]oxaborol-1(3H)-ol with potassium carbonate in tetrahydrofuran at 20;
Stage #2:2-trifluoromethyl-4-fluorobenzoyl chloride in tetrahydrofuran at 20; for 24.1667 h;Product distribution / selectivity;
Steps:
82
4-Fluoro-N-(l-hvdroxy-3,3-dimethyl-l,3-dihvdro-benzofcJfl,2Joxaborol-6-yl-2- triβuoromethyl benzatnide[0387] A lL three-necked round-bottomed-flask equipped with a nitrogen inlet adapter, mechanical stirrer and thermocouple was charged with 82e (15.7g, 88.4 mmol), THF (160 mL, anhydrous, stabilizer free) and K2CO3 (14.7g, 106 mmol). The suspension was stirred at rt and 4-fluoro-2-(trifluoromethyl)benzoyl chloride (22.Og, 97.3 mmol) was added over 10 min. The resulting suspension was aged for 24 h at rt. Water (80 mL) and isopropyl acetate (160 mL) were added and the phases were partitioned. The organic phase was further extracted with water (80 mL) and then brine (50 mL). The organic phase was dried over MgSO4 (20 g) and concentrated in vacuo to provide a tan solid (34.26 g). The solid was dissolved with acetone (195 mL) and transferred to a mechanically stirred IL round-bottomed-flask. Distilled water (113 mL) was added in one portion and the mixture was stirred for 30 min to produce a seed bed and then additional distilled water (60 mL) was added over 30 min. The suspension was stirred at rt overnight and the solids were collected on a frit. The cake was rinsed with 1 : 1 acetone/water (100 rnL) and air dried to constant weight to provide an off-white solid (30.5 g, 94%).
References:
ANACOR PHARMACEUTICALS, INC.;CHEN, Daitao;ORR, Matthew;SLIGAR, Jessica;JACOBS, Robert;PLATTNER, Jacob, J. WO2011/19618, 2011, A1 Location in patent:Page/Page column 139-140
![6-aMino-3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/20150408/GIF/1266084-49-4.gif)
1266084-49-4
9 suppliers
inquiry

189807-21-4
102 suppliers
$10.00/1g
![4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-(trifluoromethyl)benzamide(WXG02326)](/CAS/20180629/GIF/1266084-51-8.gif)
1266084-51-8
28 suppliers
inquiry
244205-40-1
320 suppliers
$9.00/1g
![4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-(trifluoromethyl)benzamide(WXG02326)](/CAS/20180629/GIF/1266084-51-8.gif)
1266084-51-8
28 suppliers
inquiry
![3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/GIF/221352-10-9.gif)
221352-10-9
76 suppliers
$40.00/100mg
![4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-(trifluoromethyl)benzamide(WXG02326)](/CAS/20180629/GIF/1266084-51-8.gif)
1266084-51-8
28 suppliers
inquiry
![3,3-diMethyl-6-nitrobenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/20150408/GIF/1266084-47-2.gif)
1266084-47-2
19 suppliers
$45.00/2.5mg
![4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-(trifluoromethyl)benzamide(WXG02326)](/CAS/20180629/GIF/1266084-51-8.gif)
1266084-51-8
28 suppliers
inquiry