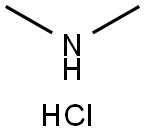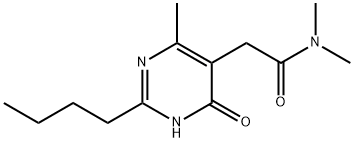
2-(2-butyl-4-hydroxy-6-MethylpyriMidin-5-yl)-N,N-diMethylacetaMide synthesis
- Product Name:2-(2-butyl-4-hydroxy-6-MethylpyriMidin-5-yl)-N,N-diMethylacetaMide
- CAS Number:1315478-13-7
- Molecular formula:C13H21N3O2
- Molecular Weight:251.32
Yield:1315478-13-7 93%
Reaction Conditions:
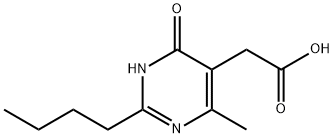
Stage #1: 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidin-5-yl)acetic acidwith triethylamine in dichloromethane at 0; for 0.5 h;
Stage #2: N,N-dimethylammonium chloridewith chloroformic acid ethyl ester in dichloromethane; for 12 h;Reflux;
Stage #3: with sodium hydroxide in dichloromethane;water; pH=9;
Steps:
3
Example 3: Preparation of 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidin-5-yl)-N,N-dimethylacetamide 5 g (22.3 mmol) of 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidin-5-yl)-acetic acid prepared in Preparation Example 3 and 7.8 mL (55.7 mmol) of triethylamine were dissolved in 100 mL of dichloromethane, followed by cooling to 0°C and stirring for 30 minutes. To the resulting solution was added dropwise 5.5 mL (55.7 mmol) of ethyl chloroformate for 5 minutes, and the reaction temperature was adjusted to 25°C, followed by stirring for 30 minutes. To the solution were added 5.5 g (66.9 mmol) of dimethylamine hydrochloride and 9.5 mL (66.9 mmol) of triethylamine, followed by reflux at 55°C for 12 hours. Then, the solution was cooled to 25°C, and 50 mL of purified water was added thereto. The solution was basified to make a pH of 9 by adding a 1N sodium hydroxide solution. The organic layer was separated, and the aqueous layer was extracted with 50 mL of dichloromethane. The combined organic layer was washed with 50 mL of brine and then dried over anhydrous sodium sulfate. After filtration, concentration was carried out under reduced pressure. The concentrate was crystallized using ethyl acetate and n-hexane to afford 5.2 g (yield: 93%) of a white title compound. 1H-NMR (400MHz, CDCl3)d 0.93 (t,3H), 1.40 (m,2H), 1.72 (m,2H), 2.34 (s,3H), 2.62 (t,2H), 2.96 (s,3H), 3.16 (s,3H), 3.56 (s,2H)
References:
WO2011/90323,2011,A2 Location in patent:Page/Page column 9; 10
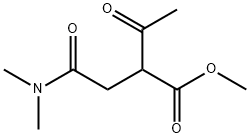
1315478-14-8
1 suppliers
inquiry

18257-46-0
66 suppliers
$14.00/1g

1315478-13-7
95 suppliers
inquiry
1315478-16-0
31 suppliers
inquiry

124-40-3
498 suppliers
$18.00/100ml

1315478-13-7
95 suppliers
inquiry
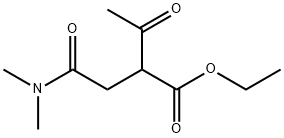
1315478-15-9
0 suppliers
inquiry

18257-46-0
66 suppliers
$14.00/1g

1315478-13-7
95 suppliers
inquiry
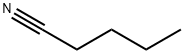
110-59-8
262 suppliers
$9.00/1g

1315478-13-7
95 suppliers
inquiry