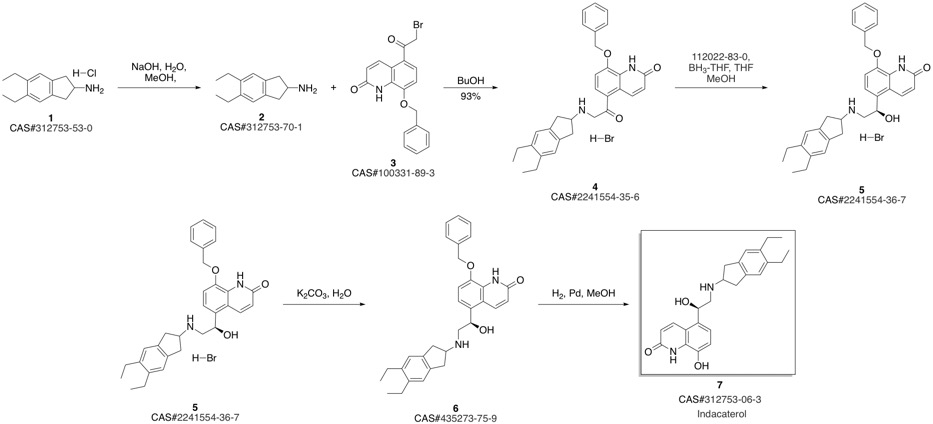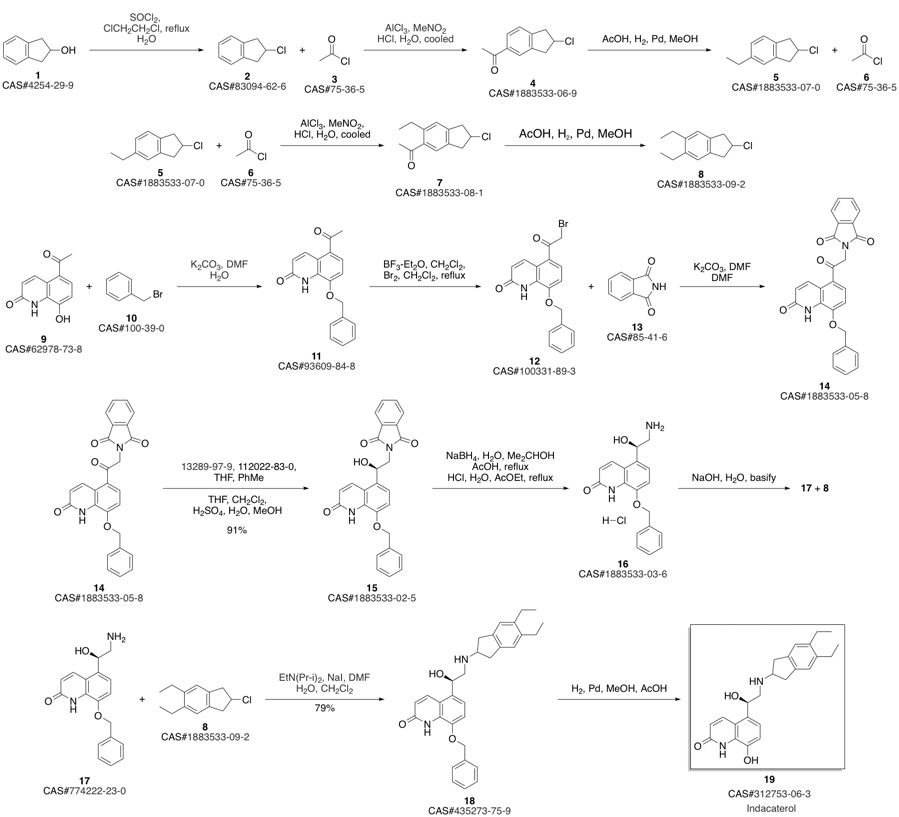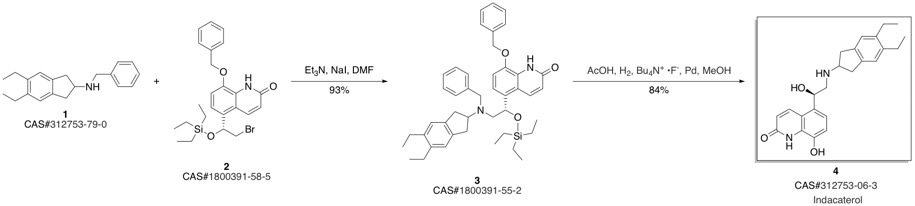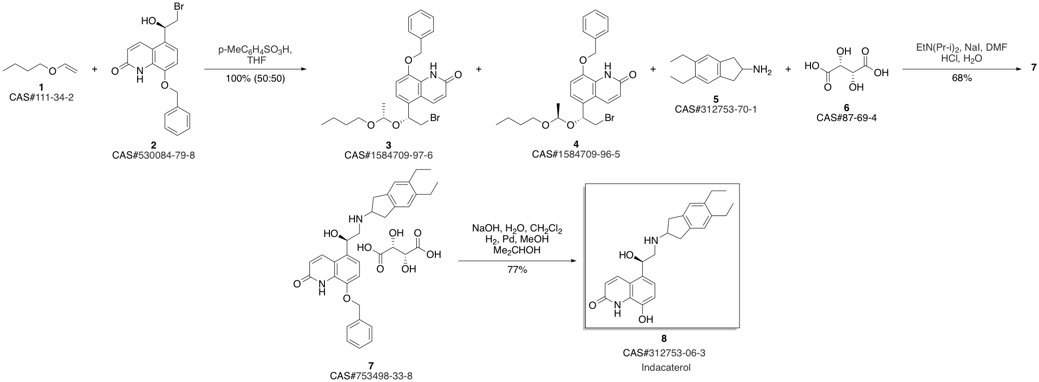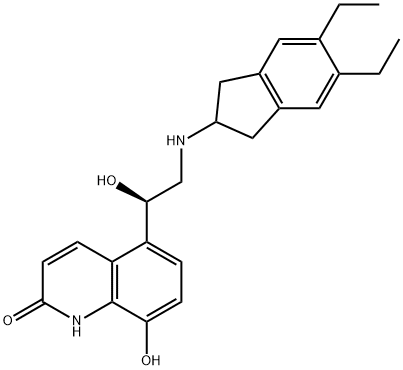
Indacaterol synthesis
- Product Name:Indacaterol
- CAS Number:312753-06-3
- Molecular formula:C24H28N2O3
- Molecular Weight:392.49
Baur, Francois; Beattie, David; Beer, David; Bentley, David; Bradley, Michelle; Bruce, Ian; Charlton, Steven J.; Cuenoud, Bernard; Ernst, Roland; Fairhurst, Robin A.; Faller, Bernard; Farr, David; Keller, Thomas; Fozard, John R.; Fullerton, Joe; Garman, Sheila; Hatto, Julia; Hayden, Claire; He, Handan; Howes, Colin; Janus, Diana; Jiang, Zhengjin; Lewis, Christine; Loeuillet-Ritzler, Frederique; Moser, Heinz; Reilly, John; Steward, Alan; Sykes, David; Tedaldi, Lauren; Trifilieff, Alexandre; Tweed, Morris; Watson, Simon; Wissler, Elke; Wyss, Daniel. The Identification of Indacaterol as an Ultralong-Acting Inhaled β2-Adrenoceptor Agonist. Journal of Medicinal Chemistry. Volume 53. Issue 9. Pages 3675-3684. Journal. (2010).
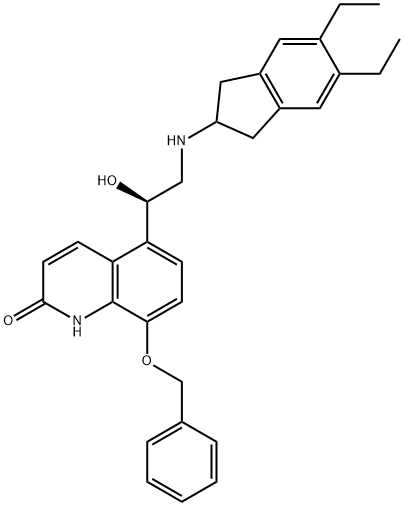
435273-75-9
87 suppliers
inquiry

312753-06-3
169 suppliers
$29.00/10mg
Yield:312753-06-3 97%
Reaction Conditions:
Stage #1: (R)-8-(benzyloxy)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-indole-2-yl)amino]-1-hydroxyethyl]quinolin-2(1H)-onewith 5%-palladium/activated carbon;hydrogen in ethanol at 20; for 2 h;
Stage #2: with 5%-palladium/activated carbon;hydrogen in ethanol at 40; under 757.576 Torr; for 4 h;
Steps:
10 Example 10. Preparation of indacaterol ((R)-2)
A mixture of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)- 1 -hydroxyethylj-8-benzyloxy-( 1H)- quinolin-2-one ((R)-1) (0.42 g, HPLC enantiomeric purity of 99.2% ee), ethanol (50 ml) andRaney nickel (0.5 g) was stirred at 20°C for 2 h. The mixture was filtered and 5% Pd / C (0.05 g) was added to the filtrate. The mixture was stirred under a hydrogen atmosphere at 40°C at the pressure of 101 kPa for 4 h. A TLC analysis of the mixture showed the pure product, therefore the mixture was hot filtered and the residue on the filter was extensively washed with hot ethanol. The filtrate was evaporated in an evaporator at a reduced pressure. The yield was0.33 g (97%) of white powder. HPLC enantiomeric purity 99.0% ee.
References:
WO2014/139485,2014,A1 Location in patent:Page/Page column 10; 11

753498-33-8
1 suppliers
inquiry

312753-06-3
169 suppliers
$29.00/10mg

312753-70-1
37 suppliers
inquiry

312753-06-3
169 suppliers
$29.00/10mg
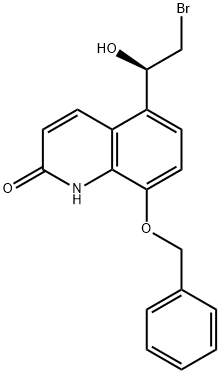
530084-79-8
137 suppliers
$16.00/100mg

312753-06-3
169 suppliers
$29.00/10mg
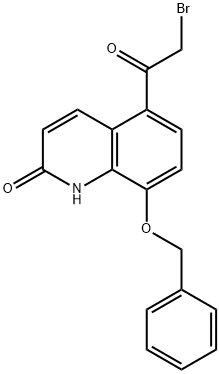
100331-89-3
201 suppliers
$70.00/10mg

312753-06-3
169 suppliers
$29.00/10mg