
5-Isoxazolecarboxylic acid, 3-(1,1-dimethylethyl)-, methyl ester synthesis
- Product Name:5-Isoxazolecarboxylic acid, 3-(1,1-dimethylethyl)-, methyl ester
- CAS Number:133674-39-2
- Molecular formula:C9H13NO3
- Molecular Weight:183.2
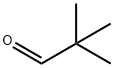
630-19-3
308 suppliers
$13.00/1g
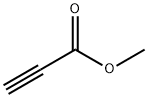
922-67-8
341 suppliers
$6.00/1g

133674-39-2
21 suppliers
$45.00/50mg
Yield: 28%
Reaction Conditions:
Stage #1:pivalaldehyde with hydroxylamine hydrochloride;sodium hydroxide in water;tert-butyl alcohol at 20; for 0.5 h;
Stage #2:propynoic acid methyl ester with chloroamine-T;copper;copper(II) sulfate in water;tert-butyl alcohol at 20;Reflux;
Steps:
D-1.1
The title compound is prepared from commercially available materials by those skilled in the art by adaptation of a literature reference (Hansen et al, J. Org. Chem., 2005, 70, 19, 7761-4).
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG WO2009/140089, 2009, A2 Location in patent:Page/Page column 47-48
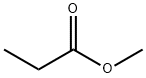
554-12-1
238 suppliers
$17.48/5 mL

630-19-3
308 suppliers
$13.00/1g

133674-39-2
21 suppliers
$45.00/50mg
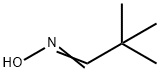
637-91-2
21 suppliers
$45.00/10mg

922-67-8
341 suppliers
$6.00/1g

133674-39-2
21 suppliers
$45.00/50mg

637-91-2
21 suppliers
$45.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

133674-39-2
21 suppliers
$45.00/50mg

630-19-3
308 suppliers
$13.00/1g

133674-39-2
21 suppliers
$45.00/50mg