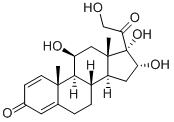
11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione synthesis
- Product Name:11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
- CAS Number:13951-70-7
- Molecular formula:C21H28O6
- Molecular Weight:376.44
86401-80-1
142 suppliers
$125.00/250mg

13951-70-7
324 suppliers
$28.00/1g
Yield:13951-70-7 93.5%
Reaction Conditions:
with sodium hydroxide in methanol;dichloromethane at 0 - 5; for 1 h;Inert atmosphere;
Steps:
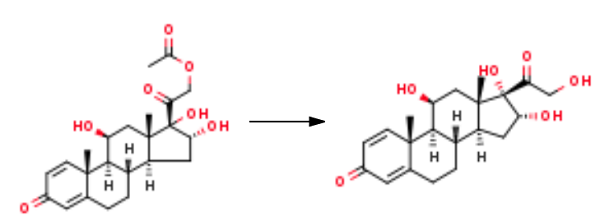
1.IV Stage-IV (Deacetylation)
Stage-IV (Deacetylation) Charge 5.83L of methanol (10 volume) and 5.83L of methylene chloride (10 volume) in a glass flask and added 583 gm of 16-HPN acetate(1.397mol) at RT. Start argon gas purging and cool to 0°C to 5°C under argon purging. Prepare 11.66 gm of sodium hydroxide (0.2915mol) solution in 0.583L of methanol (one volume) under argon purging and cool to 0°Cto 5°C. Sodium hydroxide solution is charge in reaction mass at 0°C to 5°C. Maintained the reaction mass at 0°C to 5°C for one hour, In-process check by TLC against 16-HPN acetate it should be nil. Adjust pH to neutral by 21.40ml of acetic acid (0.3742 mol); distill under reduced pressure while maintaining temperature below 40°C, till dry. Cool to ambient temperature and added 1.166L of purified water (02 volume). Cool to 0°C and maintain for one hour. Filter and wash with 300ml of purified water. Dry at 60°C (+5°C) till moisture content less than 1.0%, Yield=490gm (93.50%), HPLC Purity=98.97%, Single impurity= 0.40%.
References:
WO2016/120891,2016,A1 Location in patent:Page/Page column 13; 14
3103-17-1
35 suppliers
inquiry

13951-70-7
324 suppliers
$28.00/1g
98422-55-0
1 suppliers
inquiry

13951-70-7
324 suppliers
$28.00/1g

3044-42-6
56 suppliers
$49.00/1g

13951-70-7
324 suppliers
$28.00/1g
77017-20-0
40 suppliers
$640.00/10mg

13951-70-7
324 suppliers
$28.00/1g