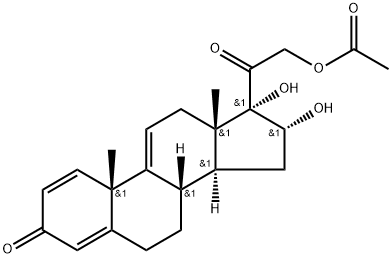
16alpha,17,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione 21-acetate synthesis
- Product Name:16alpha,17,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione 21-acetate
- CAS Number:77017-20-0
- Molecular formula:C23H28O6
- Molecular Weight:400.4648

37413-91-5
197 suppliers
$50.00/100mg

77017-20-0
45 suppliers
$640.00/10mg
Yield:77017-20-0 100%
Reaction Conditions:
Stage #1: 21-hydroxypregna-1,4,9(11),16-tetraene-3,20-dione 21-acetatewith formic acid in propan-2-one at 0; for 0.25 h;
Stage #2: with potassium permanganate in propan-2-one at -5 - 5; for 1.5 h;Temperature;
Steps:
1-3
Oxidation reaction: add 20g of tetraene acetate into the reaction flask,600mL acetone,Cool down to 0,Add 6mL formic acid,After 15 minutes of incubation reaction,Add 100 mL of 18% potassium permanganate solution to the reaction vessel,The control temperature of the dripping process does not exceed 5,The dripping is completed within 30 minutes, and the temperature is kept at 0±5 and reacted for 1 hour.After thin layer analysis to no raw material,Then add 80 mL of reducing solution (10% sodium carbonate aqueous solution) into the reactor,Stir and react for 10 minutes,The reaction system was heated to 35°C and filtered,Concentrate the filtrate until there is no acetone smell,Then add 900 mL of water to the filtrate,Cool down to below 5,Let stand for 1 hour,Suction filtration and drying to obtain 20 g of oxide (1),The yield was 100%.
References:
CN113480593,2021,A Location in patent:Paragraph 0023; 0025-0027; 0031-0033; 0037-0039

37413-91-5
197 suppliers
$50.00/100mg

77017-20-0
45 suppliers
$640.00/10mg