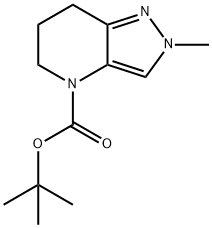
2-Methyl-2,5,6,7-tetrahydro-pyrazolo[4,3-b]pyridine-4-carboxylic acid tert-butyl ester synthesis
- Product Name:2-Methyl-2,5,6,7-tetrahydro-pyrazolo[4,3-b]pyridine-4-carboxylic acid tert-butyl ester
- CAS Number:1421312-00-6
- Molecular formula:C12H19N3O2
- Molecular Weight:237.3
![tert-butyl 6,7-dihydro-1H-pyrazolo[4,3-b]pyridine-4(5H)-carboxylate](/CAS/20180527/GIF/1569514-57-3.gif)
1569514-57-3
26 suppliers
$250.00/0.5g

74-88-4
343 suppliers
$15.00/10g
![2-Methyl-2,5,6,7-tetrahydro-pyrazolo[4,3-b]pyridine-4-carboxylic acid tert-butyl ester](/CAS/20130318/GIF/CB42641304.gif)
1421312-00-6
11 suppliers
$320.00/0.25G
![1-Methyl-1,5,6,7-tetrahydro-pyrazolo[4,3-b]pyridine-4-carboxylic acid tert-butyl ester](/CAS/20130318/GIF/CB32641307.gif)
1421311-98-9
5 suppliers
$1195.00/2.5g
Yield:1421312-00-6 28% ,1421311-98-9 28%
Reaction Conditions:
Stage #1: tert-butyl 6,7-dihydro-1H-pyrazolo[4,3-b]pyridine-4(5H)-carboxylatewith potassium tert-butylate in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 0 - 20; for 18.5 h;
Steps:
32.3 tert-Butyl 2-methyl-6J-dihydro-2H-pyrazolor4,3-blpyridine-4(5H)-carboxylate and tert-butyl 1- methyl-6,7-dihydro-lH-pyrazolor4,3-blpyridine-4(5H)-carboxylate
A solution of ie/t-butyl 6,7-dihydro-lH-pyrazolo[4,3-b]pyridine-4(5H)-carboxylate (188 mg, 842 μηιο) in N,N-dimethylformamide (5.0 mL) was cooled at 0°C with stirring then a solution of potassium ie/t-butoxide (1M in THF, 1.18 mL, 1.18 mmol) was slowly added. After stirring for 30 min methyl iodide (167 mg, 73.7 μ, 1.18 mmol) was added. After 30 min the mixture was warmed to room temperature and stirred for an additional 18 h. The mixture was quenched with saturated NH4C1 solution in water and the product was extracted with CH2CI2 (3 x 30 mL). The combined organics were dried over magnesium sulfate then concentrated in vacuo to an off- white solid. The solid was dissolved in toluene and purified by flash chromatography (115g column, 50 μιη silica gel from Analogix, 0-20 % ethyl acetate in hexanes, 15 min) to give two alkylation products. The less polar, N-2 alkylation product, ie/t-butyl 2-methyl-6,7-dihydro-2H- pyrazolo[4,3-b]pyridine-4(5H)-carboxylate (56 mg, 28 %) was obtained as a light yellow solid 1H NMR (CHLOROFORM-d) δ: 7.22 - 7.78 (m, 1H), 3.78 (s, 3H), 3.56 - 3.70 (m, 2H), 2.71 (t, J = 6.4 Hz, 2H), 1.87 - 2.00 (m, 2H), 1.46 - 1.59 (m, 9H). MS (EI/CI) m/r. 238.1 [M + H]+. The more polar, N-l alkylated product, iert-butyl l-methyl-6,7-dihydro-lH-pyrazolo[4,3-b]pyridine- 4(5H)-carboxylate (55 mg, 28 %) was obtained as a light yellow solid. 1H NMR (CHLOROFORM-d) δ: 7.52 (br. s., 1H), 3.73 (s, 3H), 3.66 (br. s., 2H), 2.65 (t, J = 6.4 Hz, 2H), 1.91 - 2.06 (m, 2H), 1.57 (br. s., 9H). MS (EI/CI) m/z: 238.1 [M + H]+.
References:
WO2014/29732,2014,A1 Location in patent:Page/Page column 74