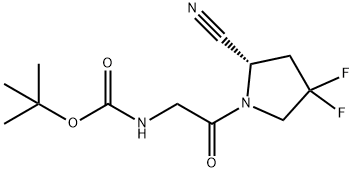
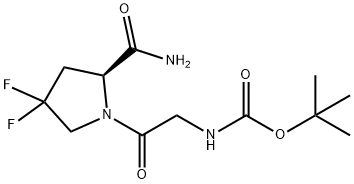
(S)-tert-butyl (2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamate synthesis
- Product Name:(S)-tert-butyl (2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamate
- CAS Number:1448440-50-3
- Molecular formula:C12H17F2N3O3
- Molecular Weight:289.28
Yield:1448440-50-3 71.68%
Reaction Conditions:
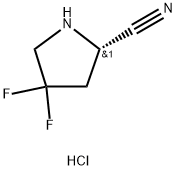
Stage #1: BOC-glycine;(S)-4,4-difluoropyrrolidine-2-carbonitrile hydrochloridewith benzotriazol-1-ol;O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate for 0.5 h;
Stage #2: with N-ethyl-N,N-diisopropylamine for 16 h;
Steps:
d
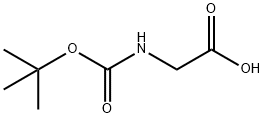
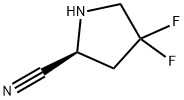
d) 2-(tert-butoxycarbonylamino)acetic acid (779.41mg, 4.45mmol, 1.5eq), 1-hydroxybenzotriazole HOBT (80.16mg, 593.22umol, 0.2eq), 2-(IH-benzotriazol-1-yl)-N,N,N',N'-tetramethylisourea phosphorus hexafluoride HBTU (2.25g, 5.93mmol, 2eq) and compounds 10 (500 mg, 2.97 mmol, 1 eq, HCl), then the reaction was stirred for 30 minutes. Then N,N-diisopropylethylamine (1.15 g, 8.90 mmol, 1.55 mL, 3 eq) was added and stirred for 16 hours. After the reaction, water (20 mL) was added and extracted with ethyl acetate (50 mL). After the organic phase was concentrated, it was purified by silica gel column chromatography (PE:ethyl acetate=1:1) to obtain compound 11 (0.625 g, 2.13 mmol, 71.68% yield).
References:
WO2022/135326,2022,A1 Location in patent:Page/Page column 13-14
1448440-48-9
10 suppliers
inquiry

1448440-50-3
22 suppliers
inquiry
203866-17-5
140 suppliers
$13.00/100mg

1448440-50-3
22 suppliers
inquiry
426844-50-0
43 suppliers
$75.00/250mg

1448440-50-3
22 suppliers
inquiry