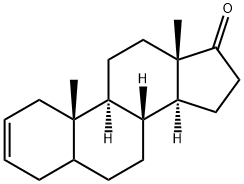
(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,5,6,7,8,9,10,11,12,13,15,16-dodecahydro-4H-cyclopenta[a]phenanthren-17(14H)-one synthesis
- Product Name:(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,5,6,7,8,9,10,11,12,13,15,16-dodecahydro-4H-cyclopenta[a]phenanthren-17(14H)-one
- CAS Number:14639-79-3
- Molecular formula:C19H28O
- Molecular Weight:272.42
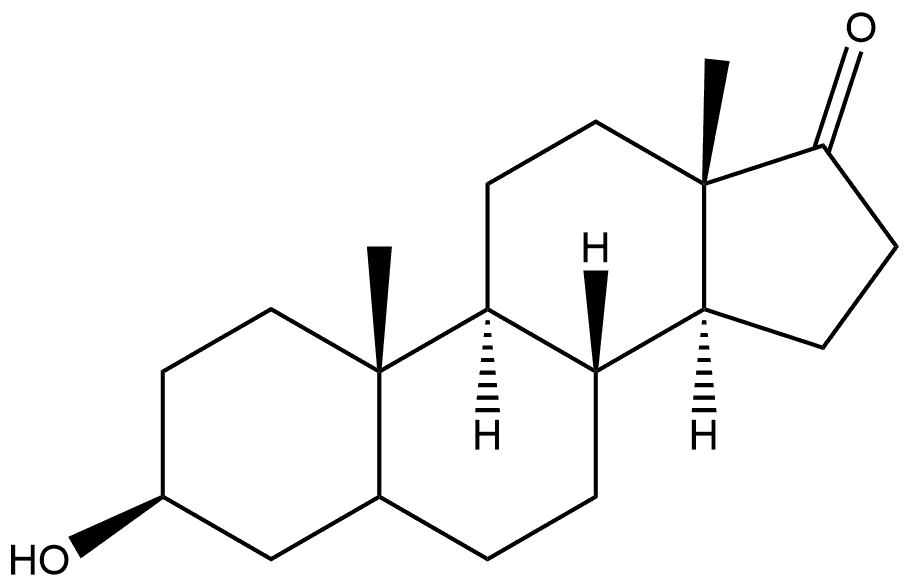
20120-08-5
0 suppliers
inquiry
![(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,5,6,7,8,9,10,11,12,13,15,16-dodecahydro-4H-cyclopenta[a]phenanthren-17(14H)-one](/CAS/20200119/GIF/14639-79-3.gif)
14639-79-3
5 suppliers
inquiry
Yield:14639-79-3 77.6%
Reaction Conditions:
in toluene at 70 - 75;Reagent/catalyst;Solvent;Temperature;
Steps:
1.C; 2.C; 3.C C. Preparation of androst-2-ene-17-one:
In a 2000 ml three-necked flask, 100 g of epiandrosterone and 1500 ml of toluene were added.The mixture was completely dissolved under stirring, and 30 g of the solid phase acid catalyst prepared in the above A was added.The reaction was stirred at 70-75 ° C for 12 to 14 hours, and the end point of the reaction was confirmed by TLC.After the reaction, the mixture was filtered under hot nitrogen, and the filter cake was washed twice with 500 ml of toluene.The solid phase acid catalyst is then recovered for use; the filtrate is combined with the wash solution.Concentrated and concentrated to recover 95% toluene, then cooled, added to pure water, and filtered.The filtrate is firstly decompressed to recover residual toluene, and then discharged into a wastewater tank for treatment. The filter cake is washed with water to obtain 96.5 g of crude crude male-2-en-17-one, and the HPLC content is 93.6%;100 g of the crude male-2-en-17-one prepared by the above method was added to 600 ml of dichloromethane andIn a mixed solvent of 400 ml of acetone, the solid is completely dissolved by heating, and then concentrated at atmospheric pressure to recover about three-quarters of a mixed solvent of dichloromethane and acetone (which can be applied to the refining process of this step).The residual liquid is slowly cooled to -5 to 0 ° C, stirred and crystallized for 4 to 5 hours, filtered, and the filtrate is used to recover the alcohol and mother liquor sleeve for the next batch of refining process; the filter cake is recrystallized again by the above recrystallization method, and the obtained filter cake is obtained. After washing and drying, 77.6 g of androst-2-ene-17-one was obtained, the HPLC content was 99.4%, the refined weight yield was 77.6%, and the total weight yield was 74.9%.
References:
CN109651473,2019,A Location in patent:Paragraph 0028; 0033-0035; 0040-0042; 0047-0048

63-05-8
291 suppliers
$32.00/1mg
![(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,5,6,7,8,9,10,11,12,13,15,16-dodecahydro-4H-cyclopenta[a]phenanthren-17(14H)-one](/CAS/20200119/GIF/14639-79-3.gif)
14639-79-3
5 suppliers
inquiry
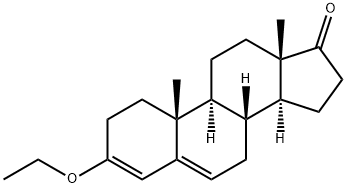
972-46-3
33 suppliers
inquiry
![(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,5,6,7,8,9,10,11,12,13,15,16-dodecahydro-4H-cyclopenta[a]phenanthren-17(14H)-one](/CAS/20200119/GIF/14639-79-3.gif)
14639-79-3
5 suppliers
inquiry