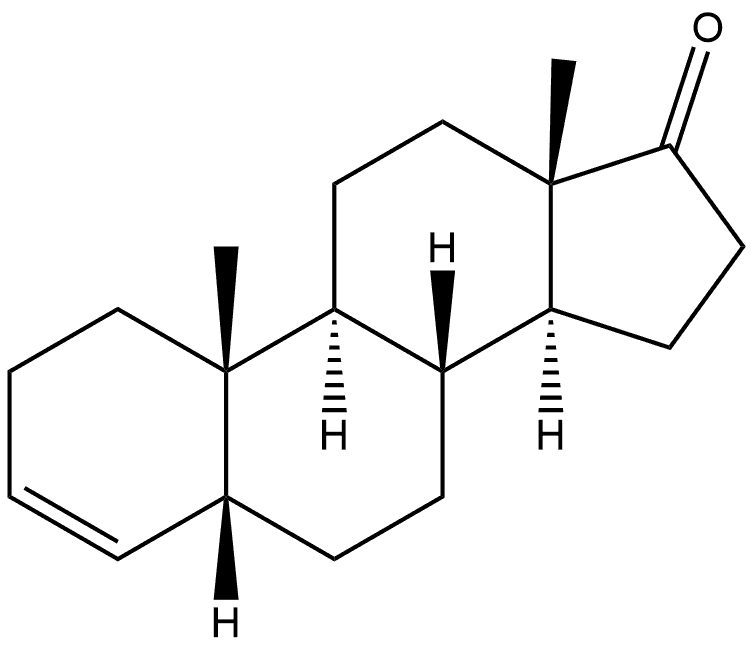
Androst-3-en-17-one,(5) synthesis
- Product Name:Androst-3-en-17-one,(5)
- CAS Number:14935-81-0
- Molecular formula:C19H28O
- Molecular Weight:272.42

63-05-8
294 suppliers
$29.00/1mg

14935-81-0
27 suppliers
inquiry
Yield: 54.94%
Reaction Conditions:
with acetic acid;zinc for 2 h;Reflux;Reagent/catalyst;
Steps:
1 Preparation of 5α-androst-3-ene-17-one
1 g of androstenedione was added to 80 mL of acetic acid, 6 g of zinc powder was added, and the mixture was heated under reflux for 2 hours. After cooling to room temperature, the reaction was stopped. After filtration, the filtrate was concentrated to dryness under reduced pressure, and methylene chloride was added. The insoluble matter was filtered off, washed with saturated aqueous sodium bicarbonate, washed with water and dried with an anhydrous sulfuric acid solution. After the filtrate was concentrated under reduced pressure ,5α-oxo-3-dilute-17-one and 5β-oxo-3-ene-17-one,Wherein the molar ratio of 5α-oxo-3-ene-17-one to 5β-oxo-3-ene-17-one is 2.3: 1,Crystallization of cyclohexane gave 5α-androst-3-ene-17-one in 0.51 g,The yield was 54.94%.
References:
Wuhan Institute of Bioengineering;Ke, xianbing;Pan, Tang CN103897013, 2016, B Location in patent:Paragraph 0019-0020
6173-23-5
0 suppliers
inquiry

14935-81-0
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14935-81-0
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14935-81-0
27 suppliers
inquiry