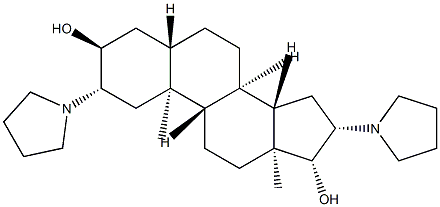
pyrrolidinyl)-, (2β,3α,5α,16β,17β)- synthesis
- Product Name:pyrrolidinyl)-, (2β,3α,5α,16β,17β)-
- CAS Number:144209-33-6
- Molecular formula:C27H46N2O2
- Molecular Weight:430.67
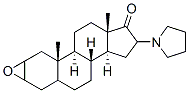
159325-45-8
42 suppliers
inquiry
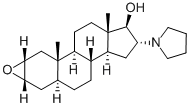
119302-19-1
81 suppliers
$421.00/100g

144209-33-6
19 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2α,3α-epoxy-16β-(1-pyrrolidinyl)-5α-androstane-17-onewith sodium tetrahydroborate in methanol;dichloromethane at 0 - 25; for 2.66667 h;
Stage #2: with water in methanol at 25; for 1 h;Product distribution / selectivity;
Steps:
28.c; 28.d
In a flask, under nitrogen atmosphere, load 8.8 g of VI (enriched isomeric ratio), 88 ml of methanol (10 vol) and 44 ml of Dichloromethane (5 vol). The mixture was then stirred at 20-25° C. until complete dissolution was achieved. The resulting solution was cooled to 0-5° C. and then 1.70 g of sodium borum hydride (45 mmols) was added portion-wise over a period of about 10 minutes. The resulting mixture was then warmed to 20-25° C. over a period of about 90 minutes and maintained with stirring for an additional 60 minutes. Then some of the solvents were distilled off under vacuum at an internal temperature of less than or equal to 40° C., until a residual volume of 79.2 ml was achieved. 35.2 ml of methanol was then added to the mixture. Then the solvents are distilled off under vacuum, until a residual volume of 79.2 ml was achieved. The mixture was then warmed to 25° C. and 352 ml of demineralized water was added under agitation over a period of about 1 hour. The resulting suspension was then cooled to -2/+2° C. over a period of at least 30 minutes, and the suspension was maintained under agitation at this temperature for an additional 90 minutes. The solid was then collected by filtration and the filter cake washed with 35 ml demineralized water. After drying (16 hrs, 40° C., under vacuum) 8.3 g of IV were obtained (yield 80.6%) This sample showed 0.83% of IV-a; 0.49% of IV-b; 1.91% of IV-c; total unknown 3.3%.; Step (d): Final Purification of Compound IV Under nitrogen, 8.3 g of IV from step (c) were loaded in a flask and, 104 ml of dichloromethane were added. The mixture was then heated to 30° C. until complete dissolution was achieved. 7.0 ml of 0.01M HCl was then added to the mixture and the mixture was stirred for 30 minutes to complete the extraction. The aqueous layer was separated from the resulting biphasic system and discarded. The organic layer was washed with 2.4 ml of 0.1M HCl, and the aqueous layer was separated and discarded. The organic layer was then washed with 2.5 ml of water, and the aqueous layer was separated and discarded. The organic layer was then heated to reflux temperature, and about 71 ml of the solvents were distilled from the organic layer over a period of about 1 hour. Then 104 ml of methanol were added. The solution was then heated to reflux temperature and about 59 ml of solvents were distilled off over a period of about 1 hour. Then, while maintaining the temperature at about 50° C., 33 ml of demineralized water were added over a period of about 15 minutes. The resulting unclear solution was then cooled to 25° C. over a period of about 1 hour. The solution was then cooled to 0/-2° C. over a period of about 1 hr and maintained at this temperature for 60 minutes. The solid was then collected by filtration and washed with 33ml of demineralized water. After drying the product for 16 Hrs at 40° C. under vacuum, 8.0 g of pure IV were obtained (IV-a 0.10% ; IV-b 0.10%; IV-c 0.58%) Yield 78%
References:
US2007/117975,2007,A1 Location in patent:Page/Page column 18

110-91-8
676 suppliers
$9.00/1g

119302-19-1
81 suppliers
$421.00/100g
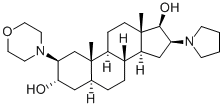
119302-20-4
245 suppliers
$34.00/250mg

144209-33-6
19 suppliers
inquiry