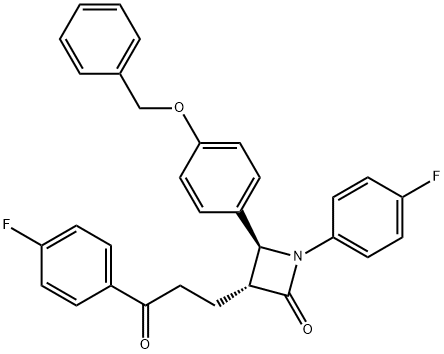
(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one synthesis
- Product Name:(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one
- CAS Number:190595-65-4
- Molecular formula:C31H25F2NO3
- Molecular Weight:497.53
![(3R,4S)-4-(4-benzyloxyphenyl)-1-(4-fluoro phenyl)-3-{2-[2-(4-fluorophenyl)-[1,3]-dioxolan-2-yl]ethyl}azetidin-2-one](/CAS/20180703/GIF/954109-22-9.gif)
954109-22-9
1 suppliers
inquiry
![(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one](/CAS/GIF/190595-65-4.gif)
190595-65-4
177 suppliers
$11.00/250mg
Yield:190595-65-4 99%
Reaction Conditions:
with water;toluene-4-sulfonic acid in acetone at 20; for 6 h;Product distribution / selectivity;Reflux;
Steps:
9 Preparation of (3R,4S)-4-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one (Compound IIa)
Example 9 Preparation of (3R,4S)-4-(4-(benzyloxy)phenyl)-1-(4-fluoro phenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one (Compound IIa) To a solution of (3R,4S)-4-(4-benzyloxyphenyl)-1-(4-fluorophenyl)-3-{2-[2-(4-fluorophenyl)-[1,3]-dioxolan-2-yl]ethyl}azetidin-2-one (7.7 g, 14.2 mmol) in wet acetone (64.7 mL acetone and 4.2 mL of water) at room temperature was added p-toluensulfonic acid monohydrate (0.27 g, 1.42 mmol). The mixture was stirred for 6 hours at reflux temperature. The solvent was then evaporated under vacuum, and ethyl acetate was added (80 mL). The organic layer was washed with 5% aqueous sodium hydrogencarbonate solution (2*80 mL) and with water (2*80 mL) and dried over sodium sulfate. The solvent was evaporated under vacuum. (3R,4S)-4-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]-azetidin-2-one was obtained as an oily product (7.24 g; 99% yield; 97% purity; 14.1 mmol).
References:
US2009/227786,2009,A1 Location in patent:Page/Page column 17
![2-Azetidinone, 1-(4-fluorophenyl)-3-[(2Z)-3-(4-fluorophenyl)-2-propen-1-yl]-4-[4-(phenylmethoxy)phenyl]-, (3R,4S)-](/CAS/20210111/GIF/1206694-77-0.gif)
1206694-77-0
0 suppliers
inquiry
![(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one](/CAS/GIF/190595-65-4.gif)
190595-65-4
177 suppliers
$11.00/250mg
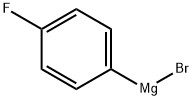
352-13-6
194 suppliers
$40.00/50mL

204589-84-4
2 suppliers
inquiry
![(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one](/CAS/GIF/190595-65-4.gif)
190595-65-4
177 suppliers
$11.00/250mg
![2-Oxazolidinone, 3-[4-[2-(4-fluorophenyl)-1,3-dioxolan-2-yl]-1-oxobutyl]-4-phenyl-, (4S)-](/CAS/20210305/GIF/942485-56-5.gif)
942485-56-5
0 suppliers
inquiry
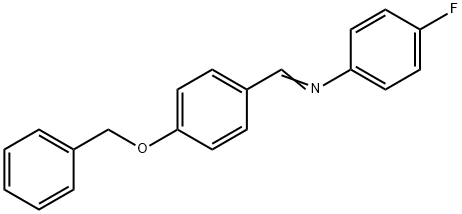
70627-52-0
209 suppliers
$5.00/1g
![(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one](/CAS/GIF/190595-65-4.gif)
190595-65-4
177 suppliers
$11.00/250mg

181705-93-1
28 suppliers
$291.89/50ml

204589-84-4
2 suppliers
inquiry
![(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one](/CAS/GIF/190595-65-4.gif)
190595-65-4
177 suppliers
$11.00/250mg