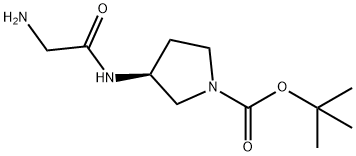
(S)-3-(2-AMino-acetylaMino)-pyrrolidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:(S)-3-(2-AMino-acetylaMino)-pyrrolidine-1-carboxylic acid tert-butyl ester
- CAS Number:193269-79-3
- Molecular formula:C11H21N3O3
- Molecular Weight:243.3
![1-Pyrrolidinecarboxylic acid, 3-[(2-bromoacetyl)amino]-, 1,1-dimethylethyl ester, (3S)-](/CAS/20210305/GIF/1055268-63-7.gif)
1055268-63-7
0 suppliers
inquiry

193269-79-3
4 suppliers
inquiry
Yield:193269-79-3 96.8%
Reaction Conditions:
with ammonium hydroxide;sodium iodide in methanol;water at 20; for 48 h;
Steps:
31.2
Step 2:To a solution of compound 157 (6.0 g, 19.53 mmol) in MeOH (110 mL) was added Nal (8.78 g, 58.6 mmol) and 28% aqueous NH4OH (1 10 mL). The resulting mixture was stirred at room temperature for 48 h. The mixture was evaporated under reduced pressure to dryness. The residue was purified by silica gel flash chromatography eluting with ACN to give 4.6 g of the desired product 158 (96.8%). 1H NMR (CDCI3, 400 MHz): δ 8.38 and 8.28 (s + s, 1H), 7.64 (br s, 2H), 4.51 - 4.42 (m, 1H), 4.25 - 4.00 (m, 2H), 3.66 - 3.34 (m, 4H), 2.20 - 2.00 (m, 2H), 1.41 (s, 9H).
References:
WO2011/160020,2011,A2 Location in patent:Page/Page column 112-113
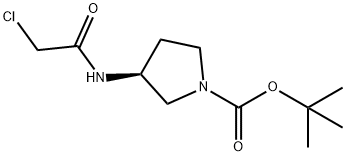
335280-33-6
8 suppliers
$322.00/250mg

193269-79-3
4 suppliers
inquiry
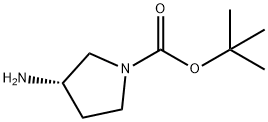
147081-44-5
323 suppliers
$14.00/1g

193269-79-3
4 suppliers
inquiry

24424-99-5
819 suppliers
$13.50/25G

193269-79-3
4 suppliers
inquiry
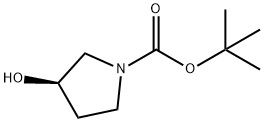
109431-87-0
354 suppliers
$5.00/1g

193269-79-3
4 suppliers
inquiry