
(1R,4R)-N-ForMyl-N-desMethyl Sertraline synthesis
- Product Name:(1R,4R)-N-ForMyl-N-desMethyl Sertraline
- CAS Number:674768-11-7
- Molecular formula:C17H15Cl2NO
- Molecular Weight:320.21

60100-09-6
0 suppliers
inquiry
155748-61-1
56 suppliers
inquiry

674768-11-7
6 suppliers
$95.00/1mg
![Formamide, N-[(1S,4R)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthalenyl]-](/CAS/20210305/GIF/674768-15-1.gif)
674768-15-1
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with formic acid at 160 - 165; for 15 h;
Steps:
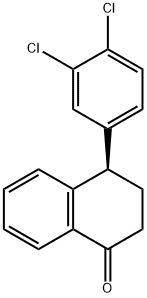
Synthesis of (1S, 4R) and [(LR,] 4R)-N- [4- (3, 4-dichlorophenyl)-1, 2,3, 4- tetrahydro-naphthalen-1-yl]-formamide
: [(R)-4- (3,] 4-dichlorophenyl) -3,4- [DIHYDRO-1-NAPHTHALENONE] (1.2 g) was added formic acid (3 mL) and formamide (3 mL). The reaction mixture was heated to 160-165° C for 15 h under nitrogen atmosphere. The reaction mixture was cooled to rt and decanted the solvent. The residue solids was passed through flash column using EtOAc: Hexane (3: 7 to 1: 1) to give the (lR, 4R)-formamide (400 mg, first spot), and the [(LS,] 4R)-formamide (360 mg). 1H NMR of the first product [[ (1R, 4R)-] isomer]: (CDCl3) 8 1.80-2. 10 (m, 3H), 2.10-2. 20 (m, [1H),] 4.00-4. 10 (m, [1H),] 5.22-5. 30 (m, 1H), 6.10-6. 20 (m, 1H), 6.80-6. 90 (M, 1H), 6.90-6. 96 (m, [1H),] 7.10-7. 40 (m, [5H),] 8.22 (s, 1H). [M+320.'H] NMR of the second product [[ (LS, 4R)-ISOMER] : [8] 1.64-1. 90 (m, 2H), 2.10-2. 28 (m, 2H), 4.10 (m, 1H), 5.38- 5.42 (m, 1H), 5.82-6. 05 (m, 1H), 6.80-6. 90 (m, 2H), 7.10-40 (m, [5H),] 8.28 (s, 1H). Mass Spec. M+ 320.
References:
WO2004/24669,2004,A1 Location in patent:Page 17