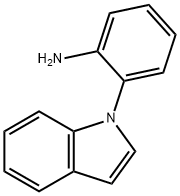
2-(1H-Indol-1-yl)aniline synthesis
- Product Name:2-(1H-Indol-1-yl)aniline
- CAS Number:473918-48-8
- Molecular formula:C14H12N2
- Molecular Weight:208.26

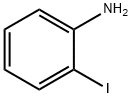
25688-25-9
8 suppliers
inquiry

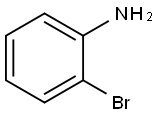
473918-48-8
10 suppliers
inquiry
Yield:473918-48-8 95%
Reaction Conditions:
with sodium tetrahydridoborate;Cupric sulfate in ethanol at 0 - 20; for 1 h;
Steps:
2-(1H-Indol-1-yl)aniline (2b) and 2-(3-Methyl-1H-indol-1-yl)ani-line (2c) 9d (Scheme 9)
CuSO 4 ·5H 2 O (4.49 g, 18 mmol) and NaBH 4 (0.85 g, 22.5 mmol) wereadded portionwise at 0 °C to a solution of 1-(2-nitrophenyl)-1H-in-dole (3.57 g, 15 mmol) or 3-methyl-1-(2-nitrophenyl)-1H-indole(3.78 g, 15 mmol) in EtOH (50 mL). The reaction mixture was thenstirred at room temperature for 1 h. The mixture was filtered througha short pad of silica gel using EtOAc (3 × 30 mL) as the eluent. The sol-vent was evaporated in vacuo and the residue was subjected to flashchromatography (petroleum ether/EtOAc, 15:1) to afford the expect-ed indole. 2-(1H-Indol-1-yl)aniline (2b)Yield: 3.17 g (95%); yellow oil.1 H NMR (400 MHz, CDCl 3 ): δ = 7.69 (dd, J = 7.2, 1.2 Hz, 1 H), 7.18 (m, 6H), 6.82 (dd, J = 7.6, 7.6 Hz, 2 H), 6.68 (d, J = 3.2 Hz, 1 H), 3.41 (br s, 2H).13 C NMR (100 MHz, CDCl 3 ): δ = 143.1, 136.4, 129.1, 128.60, 128.57,124.9, 122.2, 120.9, 120.1, 118.5, 116.2, 110.7, 103.2.
References:
Huo, Heng-Rui;Tang, Xiang-Ying;Gong, Yue-Fa [Synthesis,2018,vol. 50,# 14,p. 2727 - 2740]
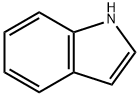
120-72-9
617 suppliers
$6.00/25g

473918-48-8
10 suppliers
inquiry

1493-27-2
504 suppliers
$5.00/10g

473918-48-8
10 suppliers
inquiry