
2-(2-aMinopyridin-4-yl)-4-chlorophenol synthesis
- Product Name:2-(2-aMinopyridin-4-yl)-4-chlorophenol
- CAS Number:1235406-61-7
- Molecular formula:C11H9ClN2O
- Molecular Weight:220.65
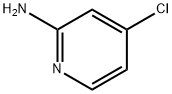
19798-80-2
530 suppliers
$6.00/1g
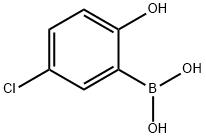
89488-25-5
128 suppliers
$12.00/100mg

1235406-61-7
9 suppliers
inquiry
Yield:1235406-61-7 82%
Reaction Conditions:
with bis-triphenylphosphine-palladium(II) chloride;sodium carbonate in 1,2-dimethoxyethane;ethanol;water at 100; for 14 h;Inert atmosphere;
Steps:
22 2-(2-aminopyridin-4-yl)-4-chlorophenol
EXAMPLE 22 Preparation of (S)-6-((l -amino- l-oxopropan-2-yl)amino)-2-(2-(2- aminopyridin-4-yl)-4-chlorophenoxy)pyrimidine-4-carboxamide (Cpd No. 67) Scheme 47 2-(2-aminopyridin-4-yl)-4-chlorophenol: A mixture of 4-chloro-2-aminopyridine (1.28 g, 10 mmol), boronic acid (1.72 g, 10 mmol), Na2C03 (3.18g, 30mmol) and Pd(PPh3)2Cl2 in DME/EtOH/H20 (4mL/2mL/4mL) was purged with Ar for one minute, then stirred at 100°C for 14 hrs. The reaction mixture was cooled to 0°C, its pH was adjusted to 5 using 6N HC1, and diluted with EtOAc. The organic layer was isolated, dried over MgS04, and concentrated under vacuum. The residue was subjected to silica gel flash chromatography using dichloromethane/methanol as the eluent to give 2-(2- aminopyridin-4-yl)-4-chlorophenol as a yellowish solid (1.8 g, yield 82%). LC/MS: m/z= 221 [M+H]+, {m/z + H) 221.
References:
WO2013/30665,2013,A1 Location in patent:Page/Page column 134; 135
84249-14-9
369 suppliers
$6.00/1g

89488-25-5
128 suppliers
$12.00/100mg

1235406-61-7
9 suppliers
inquiry