
2-(2-BROMO-5-CHLOROPHENYL)ETHAN-1-AMINE synthesis
- Product Name:2-(2-BROMO-5-CHLOROPHENYL)ETHAN-1-AMINE
- CAS Number:1379974-95-4
- Molecular formula:C8H9BrClN
- Molecular Weight:234.5208
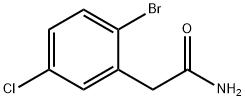
1422148-82-0
1 suppliers
inquiry

1379974-95-4
1 suppliers
inquiry
Yield:1379974-95-4 72%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 80; for 2 h;
Steps:
Two batches were run in parallel under the following conditions, then combined for purification: Borane-THF complex (1.0 M in THF, 400 mL, 400 mmol) was added dropwise to a cooled (0° C.) suspension of 2-(2-bromo-5-chlorophenyl)acetamide (Cpd AA, 22.0 g, 88.5 mmol) in anhydrous THF (300 mL). The resulting clear solution was heated to 80° C. for two hours, then cooled again to 0° C. The mixture was quenched by sequential addition of water (45 mL) and conc. HCl (120 mL), causing significant gas evolution. Stirring was continued at 10-15° C. for 16 hours, after which the mixture was concentrated to remove THF. The aqueous residue was cooled to 0° C., then 12 N aqueous sodium hydroxide was added to bring the pH to 11. The basified solution was extracted with ethyl acetate (3×500 mL). The combined organic extracts were washed with brine (500 mL), dried over anhydrous sodium sulfate, filtered, and concentrated to give crude product (25 g) as a yellow oil. Two 25 g batches of this crude product were combined, treated with 4N HCl/MeOH (500 mL), and stirred at 10-15° C. for 16 hours. The mixture was concentrated, and the residue stirred in ethyl acetate (500 mL) for 30 minutes. The resulting white solid was collected by filtration, and the filter cake washed with ethyl acetate (3×100 mL). The solids were dissolved in water (500 mL), filtered to remove insolubles, and the filtrate extracted with ethyl acetate (2×500 mL). The aqueous layer was basified with solid NaOH to pH 10, then extracted with ethyl acetate (2×500 mL). The combined organic extracts were washed with brine (500 mL), dried over anhydrous sodium sulfate, filtered, and concentrated to give 2-(2-bromo-5-chlorophenyl)ethan-1-amine (Cpd BB, 30.0 g, 72% combined yield for the two batches) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J=8.4 Hz, 1H), 7.23 (d, J=2.4 Hz, 1H), 7.07 (dd, J=2.4, 8.4 Hz, 1H), 2.98 (t, J=6.8 Hz, 2H), 2.86 (t, J=6.8 Hz, 2H), 1.28 (m, 2H).
References:
US2015/361067,2015,A1 Location in patent:Paragraph 0507
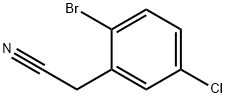
127792-49-8
34 suppliers
inquiry

1379974-95-4
1 suppliers
inquiry
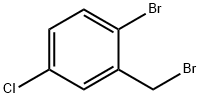
66192-24-3
119 suppliers
$9.00/1g

1379974-95-4
1 suppliers
inquiry
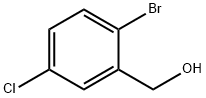
60666-70-8
90 suppliers
$9.00/1g

1379974-95-4
1 suppliers
inquiry
174265-12-4
223 suppliers
$6.00/1g

1379974-95-4
1 suppliers
inquiry