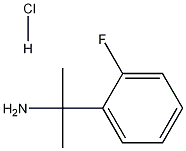
2-(2-FLUOROPHENYL)PROPAN-2-AMINE HYDROCHLORIDE synthesis
- Product Name:2-(2-FLUOROPHENYL)PROPAN-2-AMINE HYDROCHLORIDE
- CAS Number:74702-88-8
- Molecular formula:C9H12FN
- Molecular Weight:153.2

320-12-7
22 suppliers
$105.00/1g

74702-88-8
30 suppliers
$45.00/50mg
Yield:74702-88-8 410 mg
Reaction Conditions:
Stage #1: 2-(2'-fluorophenyl)propan-2-olwith trimethylsilylazide;boron trifluoride diethyl etherate in toluene at 20; for 0.25 h;
Stage #2: with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 2 h;
Steps:
2 Reference Example 2
2-(2-fluorophenyl)propane-2-amine
To a toluene solution (6 mL) of 2-(2-fluorophenyl)propane-2-ol (925 mg) while stirring, trimethylsilyl azide (830 mg) and boron trifluoride-diethyl ether complex (1021 mg) were sequentially dropped, and stirred for 15 minutes at room temperature.
After disappearance of starting materials was verified, a saturated aqueous solution of sodium hydrogen carbonate (50 mL) was added to the reaction solution, and the product was extracted with ethyl acetate (40 mL).
The organic layer was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure.
The obtained residue was dissolved in THF (15 mL), the solution was gradually dropped into a THF suspension (15 mL) of lithium aluminium hydride (228 mg) at room temperature.
After stirring for 2 hours, to the reaction solution, water (230 μL), a 2 mol/L sodium hydroxide aqueous solution (230 μL), and water (690 μL) were gradually and sequentially dropped to the reaction solution.
After being filtered with Celite, the mixture was concentrated under reduced pressure.
The obtained residue was purified with silica gel column chromatography (chloroform/methanol=92/8 to 85/15) to give 2-(2-fluorophenyl)propane-2-amine (410 mg) as an oil.
1H NMR (300 MHz, CDCl3) δ 1.55 (s, 6H), 6.99-7.12 (m, 2H), 7.18-7.27 (m, 1H), 7.41-7.46 (m, 1H)
References:
US2016/221948,2016,A1 Location in patent:Paragraph 0341-0343
445-27-2
445 suppliers
$9.00/25g

74702-88-8
30 suppliers
$45.00/50mg
394-47-8
465 suppliers
$5.00/5g

75-16-1
270 suppliers
$12.00/10ml

74702-88-8
30 suppliers
$45.00/50mg
394-35-4
209 suppliers
$6.00/5g

74702-88-8
30 suppliers
$45.00/50mg