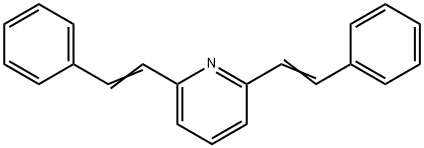
2,6-bis(2-phenylvinyl)pyridine synthesis
- Product Name:2,6-bis(2-phenylvinyl)pyridine
- CAS Number:10129-71-2
- Molecular formula:C21H17N
- Molecular Weight:283.37
Yield:10129-71-2 70%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100; for 14 h;Heck reaction;
Steps:
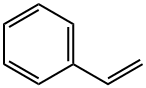
Heck coupling between styrene and different aryl halides
A typical optimization study was done by the following procedure. To a 25 mL flask was added the aryl halide, appropriate alkene and base in a mole ratio of 1:1.25:2 respectively. To this were added 5.00 mg of SnPPd and 5 mL of solvent. The reaction mixture was heated to 100 °C with stirring. The reaction was monitored by TLC. At the end of the reaction, dilute hydrochloric acid was added to the reaction mixture to quench the reaction. The product(s) was extracted with diethyl ether solvent (15 mL × 3) and the organic extract was washed with water and then with saturated sodium chloride solution. Magnesium sulfate was added to the ethereal solution to remove the water and ethereal solution was evaporated to give the crude product. This crude product was purified by column chromatography. The purity of the products was checked by proton NMR as well as by melting point measurements.; The optimized reaction conditions as found for iodobenzene-styrene coupling reactions were not very effective for the corresponding reactions involving bromobenzene. New optimized conditions for the coupling of 4-bromotoluene-styrene were found and other coupling reactions involving various aryl bromides were carried out using these conditions. In this case DMF was used as the solvent and potassium carbonate was used as the base. The temperature of the reaction was kept at 100 C. These reaction conditions were also used for arylchlorides. The results of the experiments are tabulated in Tables S3 and S4. Different aryl bromides and chlorides were reacted with styrene under the optimized conditions for 4-bromotoluene and styrene.
References:
Chandrasekhar, Vadapalli;Narayanan, Ramakirushnan Suriya [Tetrahedron Letters,2011,vol. 52,# 27,p. 3527 - 3531] Location in patent:supporting information; experimental part
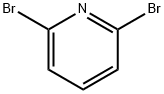
108-48-5
462 suppliers
$9.90/190μL

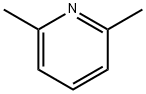
100-52-7
941 suppliers
$21.30/2g

10129-71-2
3 suppliers
inquiry
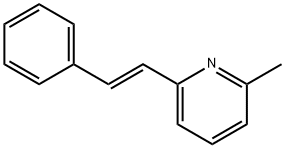
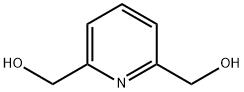
7370-21-0
11 suppliers
$140.00/10mg