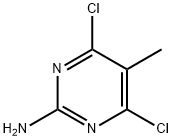
2-Amino-4,6-dichloro-5-methylpyrimidine synthesis
- Product Name:2-Amino-4,6-dichloro-5-methylpyrimidine
- CAS Number:7153-13-1
- Molecular formula:C5H5Cl2N3
- Molecular Weight:178.02
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

7153-13-1
103 suppliers
$18.00/100mg
Yield: 96%
Reaction Conditions:
with hydrogenchloride;water in ethanol at 50; for 2 h;
Steps:
3
Substituted 4,6-dichloro-2-{[(dimethylamino)methylene]amino}pyrimidines were subjected to deprotextion of the (dimethylamino)methylene protecting group from the amino group in position 2 of the pyrimidine ring. For this reaction, the method described in the literature [Nucleosides, Nucleotides & Nucleic Acids 19(1 a2), 297-327, 2000] was used, innovatively modified in order to make it possible to merely filter the product from the reaction mixture. A flask was filled with 50 mmol of substituted 4,6-dichloro-2- {[(dimethylamino)methylene] amino} -pyrimidine, 200 ml of 99% ethanol and 20 ml of resultant HC1. The reaction mixture was subsequently warmed up to 50 °C for two hours, during which a crystalline product began to separate directly from the reaction mixture. After that, 300 ml of water were added and the reaction mixture was intensively stirred for 10 minutes while quantitatively yielding the desired product, which was subsequently sucked off and rinsed with 2x 50 ml of a water/ethanol mixture (1/1), lx 5% NaHC03 aqueous solution and lx 50 ml of a water/ethanol mixture (1/1). The product was subsequently recrystallized in 99% ethanol. After complete cooling to 0 °C, the separated white crystals were sucked off, rinsed with lx 50 ml of a water/ethanol mixture (1/1) and dried in a vacuum drier.2-amino-4,6-dichloro-5-methylpyrimidine (25)Yield: 8.53 g (96 % of the theor. yield); m.p. 189-190 °C. NMR (DMSO-6): 7.26 bs, 2H, (NH2); 2.17 s, 3H, (Η-1'). 13C NMR (DMSO-6): 161.01 (C-4 and 6); 160.78 (C-2); 1 13.60 (C-5); 14.93 (C-Γ). For C5H5C12N3: calculated: 33.73 % C, 2.83 % H, 39.83 % CI, 23.60 % N; found: 33.53 % C, 2.78 % H, 40.02 % CI, 23.42 % N. MS (EI), m/z (%): 177 and 179 [M+] (100). MS (ESI+), m/z (%): 178 and 180 [M+H+] (100).
References:
USTAV ORGANICKE CHEMIE A BIOCHEMIE AKADEMIE VED CR, v.v.i.;USTAV EXPERIMENTALNI MEDICINY AKADEMIE VED CR, v.v.i.;JANSA, Petr;HOLY, Antonin;ZIDEK, Zdenek;KMONICKOVA, Eva;JANEBA, Zlatko WO2012/116666, 2012, A1 Location in patent:Page/Page column 38-39

6627-65-2
8 suppliers
inquiry

7153-13-1
103 suppliers
$18.00/100mg