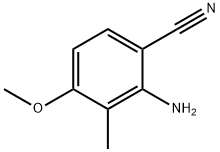
2-aMino-4-Methoxy-3-Methylbenzonitrile synthesis
- Product Name:2-aMino-4-Methoxy-3-Methylbenzonitrile
- CAS Number:923274-68-4
- Molecular formula:C9H10N2O
- Molecular Weight:162.19
Yield:923274-68-4 93%
Reaction Conditions:
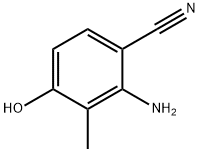
Stage #1: 2-amino-4-hydroxy-3-methylbenzonitrilewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.25 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20; for 4 h;
Steps:
77
Example 77 2-Amino-4-methoxy-3-methylbenzonitrile (77) A mixture of compound 76 (12 g, 0.08 mol), K2CO3 (11 g, 0.08 mol) in dry DMF (200 mL) was stirred for 15 min at RT. To this was added MeI (13.6 g, 0.096 mol) and the mixture was stirred for 4 h at RT. The reaction mixture was diluted with water (800 mL), extracted with 30% ethyl acetate in pet. ether (3*300 mL). The combined organic layers were washed with water and brine, dried and concentrated to give a crude product. The crude product was washed with pet. ether to give pure title compound (12 g, 93%).
References:
US2009/118312,2009,A1 Location in patent:Page/Page column 53

74-88-4
342 suppliers
$15.00/10g

923274-68-4
15 suppliers
inquiry
72817-94-8
18 suppliers
inquiry

923274-68-4
15 suppliers
inquiry
72817-85-7
23 suppliers
inquiry

923274-68-4
15 suppliers
inquiry