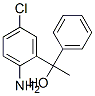
2-amino-5-chloro-alpha-methylbenzhydryl alcohol synthesis
- Product Name:2-amino-5-chloro-alpha-methylbenzhydryl alcohol
- CAS Number:3158-98-3
- Molecular formula:C14H14ClNO
- Molecular Weight:247.72
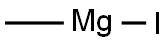
917-64-6
147 suppliers
$45.00/10ml
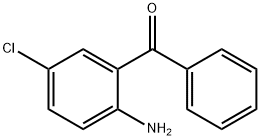
719-59-5
538 suppliers
$10.00/5g

3158-98-3
24 suppliers
$112.00/100mg
Yield:3158-98-3 530 mg
Reaction Conditions:
in diethyl ether at 0 - 20; for 5 h;Inert atmosphere;
Steps:
1 00151] Synthesis of l-(2-amino-5-chlorophenyl)-1-phenyethan-1-ol.
To 2-amino-5- chlorobenzophenone (2.34 mmols, 541 mgs) in diethyl ether (lOmL ) under nitrogen atmosphere at 0 °C was added dropwise methylmagnesium iodide (3.12 mL, 3.0M in diethyl ether). The reaction mixture was stirred and left to warm to room temperature. After 5 hours, the mixture was cooled to 0 °C and ice chips were carefully added followed by cold water. Brine was added to separate the phases. The ether layer was separated. The aqueous layer was extracted with an equal amount of ether. The combined ether layers were dried over magnesium sulfate, filtered and concentrated under vacuum to yield the tertiary alcohol (2.14 mmols, 530 mgs). MS: (M+H) 248.
References:
WO2016/154039,2016,A1 Location in patent:Paragraph 00151

75-16-1
270 suppliers
$12.00/10ml

719-59-5
538 suppliers
$10.00/5g

3158-98-3
24 suppliers
$112.00/100mg

719-59-5
538 suppliers
$10.00/5g

74-88-4
351 suppliers
$15.00/10g

3158-98-3
24 suppliers
$112.00/100mg
1685-19-4
80 suppliers
inquiry
100-58-3
192 suppliers
$18.00/10ml

3158-98-3
24 suppliers
$112.00/100mg