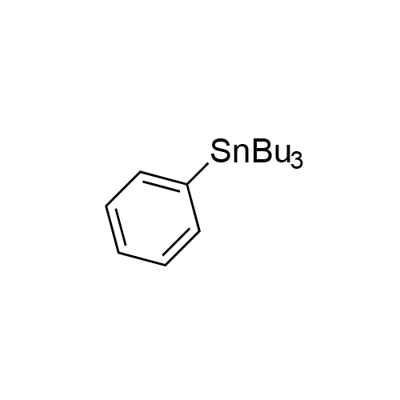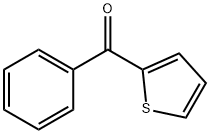
2-BENZOYLTHIOPHENE synthesis
- Product Name:2-BENZOYLTHIOPHENE
- CAS Number:135-00-2
- Molecular formula:C11H8OS
- Molecular Weight:188.25
Yield: 85%
Reaction Conditions:
with trifuran-2-yl-phosphane;palladium dichloride in chloroform at 60; for 2 h;Inert atmosphere;Stille Cross Coupling;Reagent/catalyst;
Steps:
General procedure for reaction of aryltributylstannane with aroyl chloride
General procedure: After the reaction of arylboronic acid (1 mmol) with tributyltin methoxide (0.321 g, 1 mmol) at 100 C for 1 h under solvent-free conditions, chloroform (4 mL) was added to the reaction mixture at room temperature. Either Pd(OAc)2 (0.0022 g, 0.01 mmol) or PdCl2 (0.0017 g, 0.01 mmol) and tri(2-furyl)phosphine (0.0046 g,0.02 mmol) were added under an argon gas stream, followed by addition of aroyl chloride (1 mmol) at room temperature. The resulting mixture was heated in a heating block with stirring at 60 C for 2 h. The reaction mixture was filtered through a Celite pad, and the solvent was removed under reduced pressure. After addition of THF (5 mL) and 3 M NaOH (1 mL) to the residue, the mixture was stirred for 0.5 h at room temperature and then diluted with H2O (4 mL). The aqueous phase was extracted with EtOAc (35 mL) and organic layers were washed with brine (5 mL) dried over Na2SO4, filtered, and then concentrated in vacuo. The residue was purified by column chromatography on silica gel to give pure diaryl ketone.
References:
Oikawa, Asuka;Kindaichi, Gan;Shimotori, Yasutaka;Okimoto, Mitsuhiro;Hoshi, Masayuki [Tetrahedron,2015,vol. 71,# 11,p. 1705 - 1711] Location in patent:supporting information
6165-68-0
385 suppliers
$14.00/5g
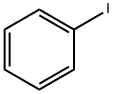
591-50-4
472 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry

135-00-2
137 suppliers
$16.00/5g
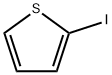
3437-95-4
244 suppliers
$6.00/5g
201230-82-2
1 suppliers
inquiry

98-80-6
712 suppliers
$5.00/5g

135-00-2
137 suppliers
$16.00/5g
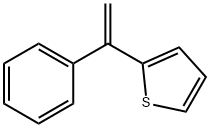
30616-74-1
0 suppliers
inquiry

135-00-2
137 suppliers
$16.00/5g