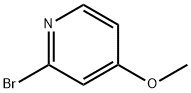
2-Bromo-4-methoxypyridine synthesis
- Product Name:2-Bromo-4-methoxypyridine
- CAS Number:89488-29-9
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02
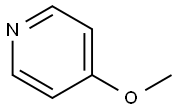
620-08-6
307 suppliers
$5.00/1g

89488-29-9
147 suppliers
$12.00/250mg
Yield: 62%
Reaction Conditions:
Stage #1:4-methoxypyridine with n-butyllithium;2-(N,N-dimethylamino)ethanol in hexane at -20; for 1 h;Inert atmosphere;
Stage #2: with 1,2-dibromo-1,1,2,2-tetrachloroethane in tetrahydrofuran;hexane at -78 - 20;Inert atmosphere;
Steps:
4-Methoxy-2-bromopyridine (2).
A solution of N,N-dimethylethanolamine (1.40 mL, 13.93 mmol) in hexanes (10 mL) at -20 oC was treated with n-BuLi (11.91 mL, 27.86 mmol, 2.34M in hexanes). The reaction mixture was stirred under nitrogen for 30 minutes. Neat 4-methoxypyridine (0.70 mL, 6.90 mmol) was added dropwise. The orange solution was stirred for one hour and then cooled to -78 oC. A solution of 1,2-dibromo-1,1,2,2-tetrachloroethane (5.40 g, 16.57 mmol) in THF (5 mL) was added dropwise, and the mixture was allowed to slowly warm to room temperature overnight. The reaction was quenched with water at 0 oC and extracted with diethyl ether. The combined extracts were dried over anhydrous magnesium sulfate and concentrated. The desired product was distilled (70 °C, 3 mmHg) to yield 0.81 g (62%) of 2 as yellow oil. 1H NMR (300 MHz, CDCl3) δ 3.86 (s, 3H), 6.78-6.80 (dd, J 2.1, 2.4, 5.9 Hz, 1H), 7.00-7.01 (d, J 2.4 Hz, 1H), 8.16-8.18 (d, J 6.0 Hz, 1H);13C NMR (75 MHz, CDCl3) δ 55.6, 110.2, 113.2, 143.0, 150.6, 166.8; FTIR (film) 2321, 2350, 2371, 2851, 2925,2956 cm-1; HRMS (FAB) calcd for C6H6BrNO (M+1) 187.9711; found 187.9708.
References:
Bori, Ibrahim D.;Comins, Daniel L. [Arkivoc,2021,vol. 2021,p. 57 - 72]

124-41-4
688 suppliers
$12.00/25g
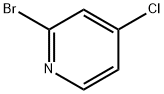
22918-01-0
264 suppliers
$6.00/1g

89488-29-9
147 suppliers
$12.00/250mg
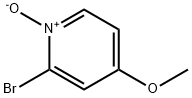
89488-33-5
1 suppliers
inquiry

89488-29-9
147 suppliers
$12.00/250mg

36953-40-9
147 suppliers
$25.00/1g

74-88-4
347 suppliers
$15.00/10g

89488-29-9
147 suppliers
$12.00/250mg
109-04-6
527 suppliers
$6.00/25g

89488-29-9
147 suppliers
$12.00/250mg