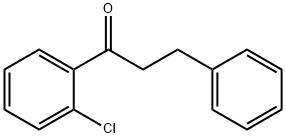
2'-CHLORO-3-PHENYLPROPIOPHENONE synthesis
- Product Name:2'-CHLORO-3-PHENYLPROPIOPHENONE
- CAS Number:898764-45-9
- Molecular formula:C15H13ClO
- Molecular Weight:244.72
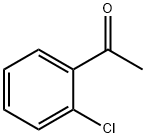
2142-68-9
388 suppliers
$6.00/10g

100-51-6
1380 suppliers
$5.00/100g

898764-45-9
9 suppliers
$433.00/1g
Yield:898764-45-9 89 %Spectr.
Reaction Conditions:
with chloro(η5-pentamethylcyclopentadienyl)(η2-pyridine-2-carboxylato)iridium(III);potassium hydroxide in toluene at 120; for 2 h;
Steps:
4.4 General procedure for the alpha alkylation of ketones with alcohols
General procedure: The reaction was carried out in Radleys Carousel 12 Plus Reaction Station. The substrates (ketone (1.0mmol)/alcohol (1.0mmol)), catalyst (0.002mmol), KOH (0.2mmol), and 1,3,5-trimethoxybenzene (used as an internal standard) were combined in an oven-dried Radleys tube. Toluene (1.0ml) was added to the tube, and the mixture was refluxed under air for 2h at 120°C. The reaction was cooled and diluted with EtOAc, and the reaction mixture was filtered through a pad of Celite. The filtrate was concentrated under reduced pressure, and the resulting residue was analyzed by 1H NMR.
References:
Pakyapan, Bilge;Kavukcu, Serdar Bat?kan;?ahin, Zarife Sibel;Türkmen, Hayati [Journal of Organometallic Chemistry,2020,vol. 925,art. no. 121486]
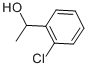
13524-04-4
120 suppliers
$44.89/5g

100-51-6
1380 suppliers
$5.00/100g

898764-45-9
9 suppliers
$433.00/1g
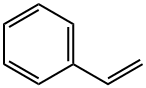
100-42-5
620 suppliers
$20.00/25mL
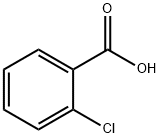
118-91-2
640 suppliers
$14.00/25g

898764-45-9
9 suppliers
$433.00/1g
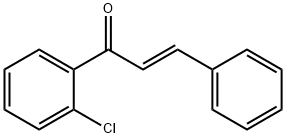
144017-77-6
3 suppliers
inquiry

898764-45-9
9 suppliers
$433.00/1g

100-42-5
620 suppliers
$20.00/25mL
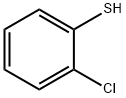
6320-03-2
147 suppliers
$12.00/1g

898764-45-9
9 suppliers
$433.00/1g