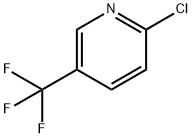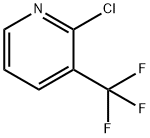
2-Chloro-3-(trifluoromethyl)pyridine synthesis
- Product Name:2-Chloro-3-(trifluoromethyl)pyridine
- CAS Number:65753-47-1
- Molecular formula:C6H3ClF3N
- Molecular Weight:181.54
2-Chloro-3-trifluoromethylpyridine is a heterocyclic organic compound and can be used as a pharmaceutical intermediate. The mixtrue of 3-chloropyridine-2-carboxylic acid,Triethylamine , NaCl , AIBN in DMF was stirred for 20 h. The reaction solution was purified to give 2-Chloro-3-(trifluoromethyl)pyridine.
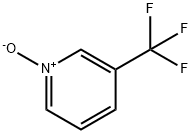
22253-72-1
4 suppliers
inquiry

65753-47-1
367 suppliers
$8.00/5g
Yield:65753-47-1 91.89%
Reaction Conditions:
Stage #1:3-(trifluoromethyl)pyridine-N-oxide with trichlorophosphate in 1,2-dichloro-ethane at -30 - -20; for 1 h;
Stage #2: with triethylamine in 1,2-dichloro-ethane at -30 - -20; for 3 h;Product distribution / selectivity;
Steps:
1.2
Example 2 In a four-necked flask equipped with a stirrer, a thermometer, and a drying tube, 32.62 g of 3-trifluoromethylpyridine N-oxide and 46.0 g of phosphorus oxychloride were charged, and the contents were allowed to react at from 105 to 110° C. for 2 hours. Subsequently, the contents were further allowed to react at from 120 to 125° C. for 5 hours. The reaction mixture was analyzed by means of liquid chromatography and confirmed to contain 0.16% of 3-trifluoromethylpyridine N-oxide, 50.34% of 2-chloro-3-trifluoromethylpyridine, and 25.34% of 2-chloro-5-trifluoromethylpyridine. The reaction mixture was heated under a reduced pressure (100 mmHg) until the internal temperature reached 75° C., thereby distilling off excessive phosphorus oxychloride. The reaction mixture was added to 163.1 g of ice water and stirred at not higher than 30° C. for one hour. Thereafter, the resultant was extracted with 1,2-dichloroethane and stirred for 30 minutes to conduct liquid separation. The obtained organic layer was washed with water to obtain 132.11 g of a 1,2-dichloroethane solution containing 2-chloro-3-trifluoromethylpyridine.
References:
ISHIHARA SANGYO KAISHA, LTD. US2012/259125, 2012, A1 Location in patent:Page/Page column 3
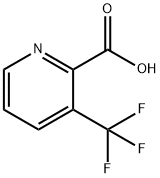
87407-12-3
145 suppliers
$8.00/250mg

65753-47-1
367 suppliers
$8.00/5g
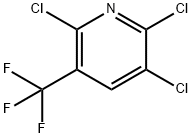
80289-91-4
37 suppliers
inquiry
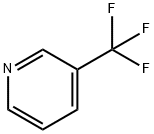
3796-23-4
219 suppliers
$7.00/1g
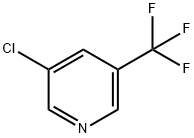
85148-26-1
200 suppliers
$32.00/1g

65753-47-1
367 suppliers
$8.00/5g
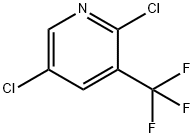
70158-59-7
85 suppliers
$31.00/100mg
108-99-6
318 suppliers
$9.00/5g

65753-47-1
367 suppliers
$8.00/5g