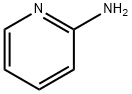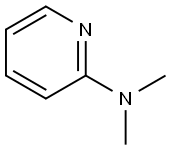
2-Dimethylaminopyridine synthesis
- Product Name:2-Dimethylaminopyridine
- CAS Number:5683-33-0
- Molecular formula:C7H10N2
- Molecular Weight:122.17
Yield:5683-33-0 55%
Reaction Conditions:
Stage #1:2-aminopyridine;formaldehyd with sodium cyanoborohydride in water;acetonitrile at 0; for 0.166667 h;
Stage #2: with acetic acid in water;acetonitrile at 20; for 15 h;
Stage #3: with sodium hydroxide in water
Steps:
9.E.1
Dimethyl-pyridin-2-yl-amineTo an ice-cold solution of 2-aminopyridine (5.0 g, 53.12 mmol) in acetonitrile (150.0 mL) was added sequentially water (33.0 mL) followed by formaldehyde (37% aq. solution, 50.0 mL) and sodium cyanoborohydride (10.0 g, 159.13 mmol). The resulting reaction mixture was stirred at O0C for 10 min followed by drop wise addition of acetic acid (12.0 mL). The reaction mixture was then allowed to stir at room temperature for 15 h. After the completion of the reaction (TLC monitoring), the solvent was evaporated and the residue was treated with aqueous NaOH (2N, 50.0
References:
PROLYSIS LTD. WO2007/148093, 2007, A1 Location in patent:Page/Page column 68-69
4597-87-9
168 suppliers
$17.00/1g

124-38-9
122 suppliers
$175.00/23402

5683-33-0
150 suppliers
$25.60/5g
109-09-1
11 suppliers
$10.60/1gm:

124-40-3
498 suppliers
$18.00/100ml

5683-33-0
150 suppliers
$25.60/5g
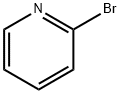
109-04-6
525 suppliers
$6.00/25g

124-40-3
498 suppliers
$18.00/100ml

5683-33-0
150 suppliers
$25.60/5g
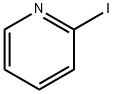
5029-67-4
294 suppliers
$6.00/1g

124-40-3
498 suppliers
$18.00/100ml

5683-33-0
150 suppliers
$25.60/5g