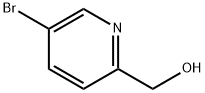
5-Bromo-2-hydroxymethylpyridine synthesis
- Product Name:5-Bromo-2-hydroxymethylpyridine
- CAS Number:88139-91-7
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02

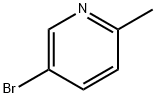
3430-13-5
418 suppliers
$5.00/5g

88139-91-7
260 suppliers
$11.00/1g
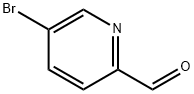
Yield:88139-91-7 78%
Reaction Conditions:
Stage #1:5-bromo-2-methylpyridine with 3-chloro-benzenecarboperoxoic acid in dichloromethane for 2 h;
Stage #2: with sodium carbonate
Steps:
413.A
Part A: Compound 413 (3.5 g, 20.7 mmol) was dissolved in methylene chloride (100 ml) and m-CPBA (4.95 g, 28.9 mmol) was added. The reaction mixture was stirred for two hours and then quenched with saturated sodium carbonate and stirred overnight. The organic layer was dried over sodium sulfate and the concentrated to provide a yellow solid (3.8 g). The solid was placed under argon and trifluoroacetic anhydride (15 mL) was added slowly. The reaction was stirred for 30 minutes at room temperature and then refluxed for 30 minutes. The reaction was cooled to room temperature and quenched slowly with saturated sodium bicarbonate. Methylene chloride was added and the organic layer was washed dried over sodium sulfate and concentrated. Column chromatography (2 to 1 ethyl acetate/hexanes) provided the desired product (3.O g, 78%).1H NMR (400 MHz, CDCI3) δ 8.65 (d, 1H), 7.8 (m, 1H), 7.2 (d, 1H), 4.7 (s, 2H).
References:
SCHERING CORPORATION WO2007/84451, 2007, A1 Location in patent:Page/Page column 146
31181-90-5
331 suppliers
$5.00/250mg

88139-91-7
260 suppliers
$11.00/1g
31181-64-3
85 suppliers
$60.00/100mg

88139-91-7
260 suppliers
$11.00/1g
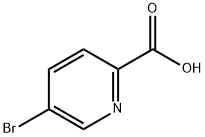
30766-11-1
395 suppliers
$3.00/1g

88139-91-7
260 suppliers
$11.00/1g
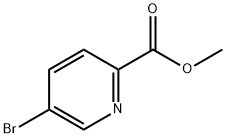
29682-15-3
270 suppliers
$8.00/1g

88139-91-7
260 suppliers
$11.00/1g