
2-HYDROXYTHIOANISOLE synthesis
- Product Name:2-HYDROXYTHIOANISOLE
- CAS Number:1073-29-6
- Molecular formula:C7H8OS
- Molecular Weight:140.2
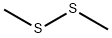
624-92-0
312 suppliers
$20.62/5 mL

10026-11-6
209 suppliers
$24.00/50g

108-95-2
723 suppliers
$14.00/25g

1073-29-6
168 suppliers
$19.00/5g
Yield:1073-29-6 39%
Reaction Conditions:
with mercury
Steps:
6 Preparation of Ortho-(Methylthio)phenol
EXAMPLE 6 Preparation of Ortho-(Methylthio)phenol Zirconium tetrachloride (23.4 g, 0.1 mole) was added to phenol (141.2 g, 1.5 moles). The mixture was slowly heated to 150° C. under nitrogen and maintained at this temperature for 12 hours during which HCl was allowed to escape. The mixture was cooled to ca. 100° C. and methyl disulfide (94.2 g, 1 mole) was added. The mixture was heated under nitrogen for 15 hours during which temperature was increased slowly to 159° C. Methyl mercaptan was allowed to escape continuously. Distillation of the mixture at 1.4 mm mercury to a maximum head temperature of 113° C. yielded 125.6 g of distillate. GC analysis indicated the distillate contained 59.1 g of phenol (42% recovered) and 54.2 g of ortho-(methylthio)phenol (39% yield). The unreacted phenol was removed from the distillate by fractional distillation through a 12 cm glass helices packed column. The remaining material was fractionally distilled through a 5 inch Vigreux column yielding a main fraction of 99.6% pure ortho-(methylthio)phenol distillating at 112°-114° C. at 30 mm mercury.
References:
E. I. DuPont de Nemours and Company US4599451, 1986, A
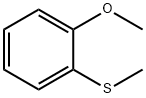
2388-73-0
106 suppliers
$12.00/1g

1073-29-6
168 suppliers
$19.00/5g
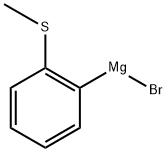
99346-84-6
13 suppliers
inquiry

1073-29-6
168 suppliers
$19.00/5g

108-95-2
723 suppliers
$14.00/25g

1073-29-6
168 suppliers
$19.00/5g
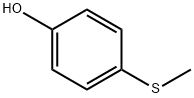
1073-72-9
221 suppliers
$6.00/1g
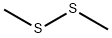
624-92-0
312 suppliers
$20.62/5 mL

108-95-2
723 suppliers
$14.00/25g

1073-29-6
168 suppliers
$19.00/5g