
2-methylsulfanylquinoline synthesis
- Product Name:2-methylsulfanylquinoline
- CAS Number:40279-26-3
- Molecular formula:C10H9NS
- Molecular Weight:175.25
Yield:40279-26-3 80%
Reaction Conditions:
in N,N-dimethyl-formamide at 120; for 12 h;Inert atmosphere;Schlenk technique;
Steps:
Preparation of 2-(methylsulfanyl)quinoline (1)
Starting material 1 was prepared according to the method described by Dahaen with a slight modification. A Schlenk tube was charged with 2-chloroquinoline (3.3 g, 20 mmol) and DMF (40 mL). Sodium methanethiolate (90 wt%, 10.3 g, 22 mmol) was added and the resulting mixture was stirred for12 h at 120 °C. The resulting biphasic solution was extracted with Et2O (40 mL × 3). The combinedorganic layer was dried over Na2SO4 and concentrated under a reduced pressure. The resulting residuewas purified by silica gel column chromatography with an eluent (hexane/AcOEt = 20/1) to give 1 (2.8 g,16 mmol, 80%) as a white solid.
References:
Yamagishi, Hiroki;Tsuchiya, Shun;Saito, Hayate;Nogi, Keisuke;Shimokawa, Jun;Yorimitsu, Hideki [Heterocycles,2019,vol. 99,# 1,art. no. A34,p. 301 - 309]

2637-37-8
52 suppliers
$24.89/250mg

74-88-4
340 suppliers
$15.00/10g

40279-26-3
11 suppliers
inquiry
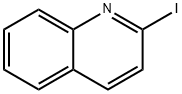
6560-83-4
40 suppliers
$160.00/250mg

75-18-3
386 suppliers
$19.00/25mL

40279-26-3
11 suppliers
inquiry
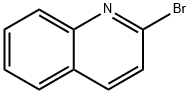
2005-43-8
200 suppliers
$6.00/250mg
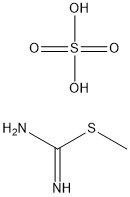
867-44-7
367 suppliers
$5.00/10g

40279-26-3
11 suppliers
inquiry
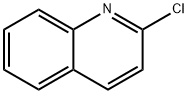
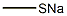
![Silane, [2,2-bis(ethylthio)ethenyl]trimethyl-](/CAS/20210305/GIF/349449-55-4.gif)