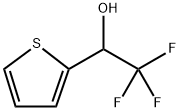
2-Thiophenemethanol, a-(trifluoromethyl)- synthesis
- Product Name:2-Thiophenemethanol, a-(trifluoromethyl)-
- CAS Number:35304-68-8
- Molecular formula:C6H5F3OS
- Molecular Weight:182.16
Yield:35304-68-8 97%
Reaction Conditions:
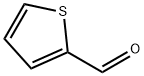
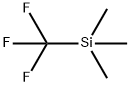
Stage #1: thiophene-2-carbaldehyde;(trifluoromethyl)trimethylsilanewith cesium fluoride in 1,2-dimethoxyethane at 0 - 25;
Stage #2: with hydrogenchloride in 1,2-dimethoxyethane;water; for 0.5 h;
Steps:
57.1 Step 1
To a suspension of 125a (11.2 g, 0.1 mol) and CsF (1.52 g, 0.01 mol) in DME (70 mL) was added TMSCF3 (28.4 g, 0.2 mol) dropwise at 0 °C. Then the mixture was stirred at 25°C for 3 hours. The starting material was consumed up. After that, HC1 (3N) was added to quench the reaction and it was stirred for another 0.5 hours. The intermediate was used up and the desired product was formed. Extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated in vacuo and purified using Si02 chromatography (200 g, Petroleum Ether/EtOAc = 1% to 2%) to providel25b as a oil (17.2 g, 97% yield). 1H NMR (CDC13): 7.39 - 7.38 (m, 1 H) , 7.19 - 7.18 d, 1 H, J=3.6Hz), 7.05 - 7.03 (m, 1 H), 5.29 - 5.23 (m, 1 H)
References:
WO2014/110688,2014,A1 Location in patent:Page/Page column 154