
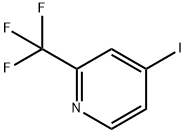
2-(Trifluoromethyl)pyridine-4-boronic acid synthesis
- Product Name:2-(Trifluoromethyl)pyridine-4-boronic acid
- CAS Number:1093407-58-9
- Molecular formula:C6H5BF3NO2
- Molecular Weight:190.92

5419-55-6
371 suppliers
$12.00/5g
590371-73-6
107 suppliers
$18.00/250mg

1093407-58-9
88 suppliers
$65.00/50mg
Yield: 67%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane at -78 - -10; for 3.5 h;Inert atmosphere;
Steps:
3 2-(Trifluoromethyl)pyridine-4-boronic acid
To a solution of 4-iodo-2-(trifluoromethyl)pyridine [purchased from Matrix Scientific] (2.5 g, 9.16 mmol) and triisopropylborate (2.54 mL, 10.99 mmol) in anhydrous tetrahydrofuran (20 mL) at -78° C. under nitrogen was added dropwise a solution of n-butyl lithium in hexane (2.5 M, 4.03 mL, 10.07 mmol).
The reaction was stirred at -78° C. for 2½ hours then allowed to warm slowly to -10° C. over 1 hour.
The reaction mixture was then quenched by the addition of water (20 mL) and the tetrahydrofuran removed under reduced pressure.
The resulting aqueous phase was diluted with water (40 mL) and washed with ether (2*40 mL).
The aqueous phase was then acidified to pH 5 by the addition of acetic acid and the resulting oily suspension extracted with ether (100 mL).
The organic phase was separated, washed with saturated brine (2*50 mL), dried (MgSO4), filtered and concentrated under reduced pressure to give an off-white foam.
Ether (12 mL) and hexanes (24 mL) were added and the resulting suspension stirred vigorously for 30 minutes then filtered to give 2-(trifluoromethyl)pyridine-4-boronic acid (1.163 g, 67% yield) as a cream solid.
HPLC/MS Rt=1.54 min, m/z 192.1 (M+H+).
References:
Jacobus Pharmaceutical Company, Inc.;Heffernan, Gavin David;Jacobus, David Penman;Saionz, Kurt William;Schiehser, Guy Alan;Shieh, Hong-Ming;Zhao, Wenyi US2014/135320, 2014, A1 Location in patent:Paragraph 0227; 0228