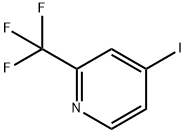
4-Iodo-2-(trifluoromethyl)pyridine synthesis
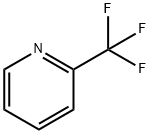
- Product Name:4-Iodo-2-(trifluoromethyl)pyridine
- CAS Number:590371-73-6
- Molecular formula:C6H3F3IN
- Molecular Weight:272.99
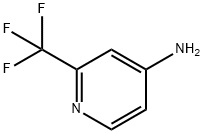
147149-98-2
229 suppliers
$30.00/1g

590371-73-6
107 suppliers
$18.00/250mg
Yield:590371-73-6 71%
Reaction Conditions:
with hydrogenchloride;sodium nitrite in water at 0 - 20; for 1.16667 h;
Steps:
8.1 Step-1: Synthesis of 4-iodo-2-(trifluoromethyl)pyridine
To a stirred solution of 2-(trifluoromethyl)pyridin-4-amine (1.0 g, 6.10 mmol, 1.0 eq.) in 5N HCl (10 mL) was added NaNO2 (0.63 g, 0.91 mmol, 1.5 eq.) drop wise dissolved in water (10 mL) at 0° C. After 10 min KI (2.1 g, 12.8 mmol, 2.1 eq.) was added drop wise dissolved in (10 mL).
The reaction mixture was stirred at same temperature for 10 min and 1 h in RT.
Following this, reaction was allowed diluted with ethyl acetate (30 mL).
The aqueous layer was separated pH of aqueous layer was adjusted ?11 by adding 6N NaOH, the layers were separated and the organic layer was washed with 0.3M Na2S2O3 (2*20 mL).
The combined organic layers were washed with brine (30 mL), dried over anhydrous Na2SO4, filtered and concentrated under vacuum to get the solid residue which was purified by was purified by normal phase silica-gel column chromatography to get the title compound as white solid (0.71 g, 71%). LCMS: 274.0 [M+1]+; 1H NMR (400 MHz, DMSO-d6) δ ppm 8.45 (d, J=4.89 Hz, 1H) 8.31 (s, 1H) 8.18 (d, J=4.89 Hz, 1H)
References:
US2020/206233,2020,A1 Location in patent:Paragraph 0249-0250
590371-71-4
44 suppliers
$180.25/1gm:

590371-73-6
107 suppliers
$18.00/250mg
131747-41-6
212 suppliers
$10.00/1g

590371-73-6
107 suppliers
$18.00/250mg
368-48-9
219 suppliers
$8.00/1g

590371-73-6
107 suppliers
$18.00/250mg