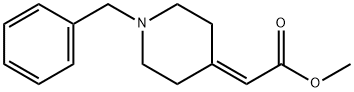
(1-Benzyl-piperidin-4-ylidene)-acetic acid Methyl ester synthesis
- Product Name:(1-Benzyl-piperidin-4-ylidene)-acetic acid Methyl ester
- CAS Number:206558-34-1
- Molecular formula:C15H19NO2
- Molecular Weight:245.32
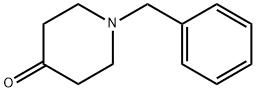
3612-20-2
485 suppliers
$6.00/10g
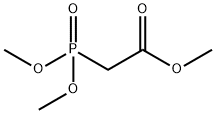
5927-18-4
318 suppliers
$5.00/25g

206558-34-1
23 suppliers
$174.00/250mg
Yield:-
Reaction Conditions:
Stage #1:trimethyl phosphonoacetate with sodium hydride in DMF (N,N-dimethyl-formamide) at 0; for 0.5 h;
Stage #2:1-phenylmethyl-4-piperidone in DMF (N,N-dimethyl-formamide) at 0 - 20; for 1 h;
Steps:
15
Sodium hydride (60% in mineral oil, 10.55 g, 264 mmol) was washed with hexanes then suspended in N,N-dimethylformamide (200 mL). Mixture was cooled to 0° C. Trimethyl phosphonoacetate (38.0 mL, 249 mmol) was added to the mixture dropwise. The reaction was stirred at 0° C. for 30 minutes. 1-Benzyl-4-piperidone (40.0 mL, 220 mmol) was added to the reaction mixture dropwise. The reaction was warmed to ambient temperature and held with stirring for 1 h. The reaction mixture was diluted with diethyl ether (500 mL), washed with water (2×), then brine. The organic layer was dried (magnesium sulfate), filtered, and concentrated in vacuo. The residue was dissolved in methanol (220 mL). Platinum(IV) oxide (600 mg, 2.64 mmol) was added to the mixture. The reaction vessel was placed on a Parr apparatus, charged with 40 psi of hydrogen gas, and shaken at room temperature for 5 h. The reaction mixture was removed from the apparatus, filtered through celite, and concentrated. The residue was passed through a short column of silica gel eluting with ethyl acetate. Fractions were concentrated in vacuo. The title compound was obtained as amber oil in 90% yield. 1H NMR (300 MHz, CDCl3): δ 7.31-7.16 (m, 5H), 3.62 (s, 3H), 3.45 (s, 2H), 2.83 (d, J=11.71, 2H), 2.20 (d, J-6.95, 2H), 2.00-1.88 (m, 1H), 1.82-1.69 (m, 1H), 1.69-1.59 (m, 2H), 1.38-1.25 (m, 2H). Mass spec.: 249.3 (MH)+.
References:
Degnan, Andrew P.;Han, Xiaojun;Dubowchik, Gene M.;Macor, John E.;Mercer, Stephen E. US2005/215576, 2005, A1 Location in patent:Page/Page column 47