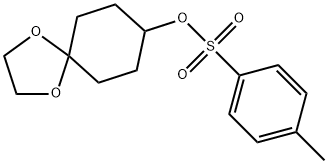
1,4-Dioxaspiro[4.5]decan-8-ol 4-methylbenzenesulfonate synthesis
- Product Name:1,4-Dioxaspiro[4.5]decan-8-ol 4-methylbenzenesulfonate
- CAS Number:23511-05-9
- Molecular formula:C15H20O5S
- Molecular Weight:312.38
Yield:23511-05-9 99%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 0 - 20; for 16 h;Inert atmosphere;
Steps:
1.1 Step 1. Preparation of l,4-dioxaspiro[4.5]decan-8-yl 4-methylbenzenesulfonate
Step 1. Preparation of l,4-dioxaspiro[4.5]decan-8-yl 4-methylbenzenesulfonate To a 0 °C solution of l,4-dioxaspiro[4.5]decan-8-ol (10 g, 63.2 mmol), triethylamine (13.22 mL, 95.0 mmol), and N,N-dimethylpyridin-4-amine (0.772 g, 6.32 mmol) in CH2CI2 (400 mL) was added portion wise 4-methylbenzene-l-sulfonyl chloride (13.26 g, 69.5 mmol). The reaction mixture was allowed to warm to room temperature while stirring for 16 hours. The reaction mixture was diluted with CH2CI2 (400 mL) and was washed with water (2 x 400 mL). The combined organic layers were dried over MgS04, filtered and concentrated. The product was purified by column chromatography on silica gel (0%→ 30% ethyl acetate in hexanes; the crude product was divided in half and purified on two 330 g columns) to afford l,4-dioxaspiro[4.5]decan-8-yl 4- methylbenzenesulfonate (19.6 g, 62.7 mmol, 99% yield): XH NMR (400MHz, CDC13) δ 7.82 (d, J=8.3 Hz, 2H), 7.35 (d, J=8.0 Hz, 2H), 4.66 (tt, J=6.0, 3.0 Hz, 1H), 3.98 - 3.88 (m, 4H), 2.47 (s, 3H), 1.95 - 1.74 (m, 5H), 1.63 - 1.51 (m, 3H); LC/MS (ESI) mle 335.2 [(M+Na)+, calcd for Ci5H2o05S a 335.1], tR = 2.06 min (method 2-1).
References:
WO2015/157483,2015,A1 Location in patent:Page/Page column 246; 247
![1,4-DIOXA-SPIRO[4.5]DECAN-8-OL](/CAS/GIF/22428-87-1.gif)
22428-87-1
178 suppliers
$6.00/1g
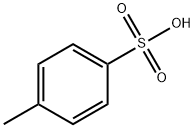
104-15-4
720 suppliers
$133.00/500g
![1,4-Dioxaspiro[4.5]decan-8-ol 4-methylbenzenesulfonate](/CAS/GIF/23511-05-9.gif)
23511-05-9
27 suppliers
$288.66/1g
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
466 suppliers
$5.00/1g
![1,4-Dioxaspiro[4.5]decan-8-ol 4-methylbenzenesulfonate](/CAS/GIF/23511-05-9.gif)
23511-05-9
27 suppliers
$288.66/1g
