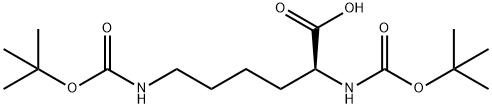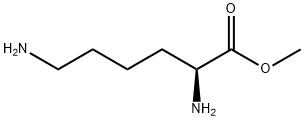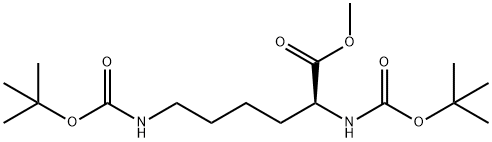
methyl (2S)-2,6-bis({[(tert-butoxy)carbonyl]amino})hexanoate synthesis
- Product Name:methyl (2S)-2,6-bis({[(tert-butoxy)carbonyl]amino})hexanoate
- CAS Number:2483-48-9
- Molecular formula:C17H32N2O6
- Molecular Weight:360.45
Yield:2483-48-9 97%
Reaction Conditions:
with potassium carbonate in ethyl acetate;N,N-dimethyl-formamide at 10 - 20; for 5 h;Inert atmosphere;
Steps:
2 Boc-L-Lys(Boc)-OMe
Example 2
Boc-L-Lys(Boc)-OMe
A 1 L three-neck flask equipped with addition funnel, nitrogen inlet, thermometer and mechanical stirrer was charged with a solution of Boc-L-Lys(Boc)-OH (59.37 g, 161 mmol, 94 wt % in EtOAc) in N,N-dimethylformamide (anhydrous) (300 mL).
Whilst stirring, potassium carbonate (24.5 g, 177 mmol) was added, and the resulting suspension was cooled on an ice bath to an internal temperature of +10° C. Iodomethane (34.3 g, 242 mmol, 15.04 mL) was added dropwise over 30 min via the addition funnel, while keeping the internal temperature <20° C.
The ice bath was removed and the reaction mixture was allowed to reach room temperature. TLC indicated complete consumption of the starting material after 4.5 h of stirring at room temperature.
All volatiles were removed in vacuo, the resulting white slurry solidified on standing, and it was stored at -20° C. overnight.
The resultant material was taken up in water (1 L) and sat'd. NaHCO3 (0.5 L), and then extracted with EtOAc (3*500 mL).
The combined organic phases were washed with dilute brine (2*200 mL), brine (2*100 mL), then dried over Na2SO4 and concentrated.
After stripping with heptane (2*200 mL), the title compound was isolated as a white solid (57.08 g, 97% yield).
1H-NMR (CDCl3):
In agreement with structure.
LCMS (NQAD detection):
Purity >99%, mass in agreement with molecular formula (pos. m/z=361 [M+1]+, 383 [M+Na]+).
Specific rotation: [α]D22=6.38° (c=1.72, CHCl3).
References:
US2016/376618,2016,A1 Location in patent:Paragraph 0213-0218

24424-99-5
821 suppliers
$13.50/25G
2389-48-2
207 suppliers
$8.00/250mg
![methyl (2S)-2,6-bis({[(tert-butoxy)carbonyl]amino})hexanoate](/CAS/20200611/GIF/2483-48-9.gif)
2483-48-9
17 suppliers
inquiry

67-56-1
739 suppliers
$9.00/25ml

56-87-1
720 suppliers
$5.00/5g

24424-99-5
821 suppliers
$13.50/25G
![methyl (2S)-2,6-bis({[(tert-butoxy)carbonyl]amino})hexanoate](/CAS/20200611/GIF/2483-48-9.gif)
2483-48-9
17 suppliers
inquiry