
(4-BROMO-BENZYL)-METHYL-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(4-BROMO-BENZYL)-METHYL-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:260809-26-5
- Molecular formula:C13H18BrNO2
- Molecular Weight:300.19
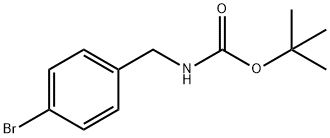
68819-84-1
118 suppliers
$11.00/250mg

74-88-4
350 suppliers
$15.00/10g

260809-26-5
28 suppliers
$98.00/50mg
Yield:-
Reaction Conditions:
with silver(l) oxide in N,N-dimethyl-formamide at 50; for 18 h;
Steps:
V.V-2.b tert-Butyl-4-bromobenzyl(methyl)carbamate
In a 60-mL vial, tert-butyl-4-bromobenzylcarbamate (1.25 g, 4.37 mmol) was dissolved in DMF (12 mL).
To this solution was added Ag2O (4.0 g, 17 mmol) followed by the addition of CH3I (0.68 mL, 11 mmol).
The mixture was stirred at 50° C. for 18 hours.
The reaction mixture was filtered through a bed of celite and the celite was washed with methanol (2*20 mL) and dichloromethane (2*20 mL).
The filtrate was concentrated to remove most of the DMF.
The residue was treated with water (50 mL) and a white emulsion formed.
This mixture was extracted with ethyl acetate (4*25 mL), dried over Na2SO4, and the solvent was evaporated to yield tert-butyl-4-bromobenzyl(methyl)carbamate as a yellow oil. 1H NMR (300 MHz, DMSO-d6) δ 7.53 (d, J=8.1 Hz, 2H), 7.15 (d, J=8.4 Hz, 2H), 4.32 (s, 2H), 2.74 (s, 3H), 1.38 (s, 9H).
References:
Vertex Pharmaceuticals Incorporated;Van Goor, Fredrick F.;Burton, William Lawrence US2015/231142, 2015, A1 Location in patent:Paragraph 1376

24424-99-5
850 suppliers
$13.50/25G
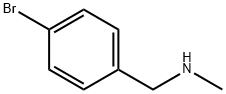
699-03-6
104 suppliers
$19.00/1g

260809-26-5
28 suppliers
$98.00/50mg

24424-99-5
850 suppliers
$13.50/25G
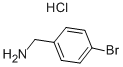
26177-44-6
180 suppliers
$6.00/1g

260809-26-5
28 suppliers
$98.00/50mg