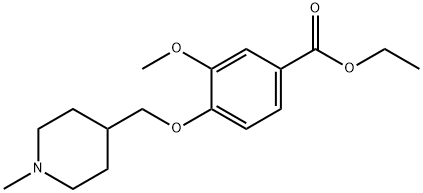
ethyl 4-((1-Methylpiperidin-4-yl)Methoxy)-3-Methoxybenzoate synthesis
- Product Name:ethyl 4-((1-Methylpiperidin-4-yl)Methoxy)-3-Methoxybenzoate
- CAS Number:264208-60-8
- Molecular formula:C17H25NO4
- Molecular Weight:307.38
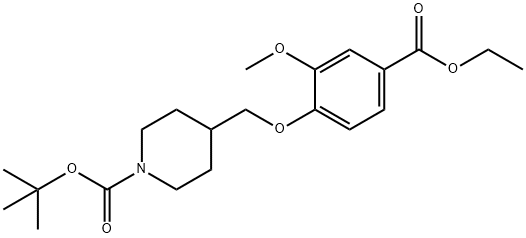
264208-58-4
8 suppliers
inquiry

264208-60-8
11 suppliers
inquiry
Yield:264208-60-8 100%
Reaction Conditions:
with hydrogenchloride;formaldehyd in formic acid;diethyl ether;dichloromethane;water;
Steps:
9.e Preparation of Compound 9 in Table 2
e) Formaldehyde (35 ml of a 37% solution in water, 420 mmol) was added to a solution of ethyl 3-methoxy-4-(1-tert-butyloxycarbonylpiperidin-4-ylmethoxy)benzoate (35 g, 89 mmol) in formic acid (35 ml) and the reaction was heated at 95° C. for 3 hours. The reaction was cooled, the volatiles we removed in vacuo and the residue was dissolved in dichloromethane. 3.0 N Hydrogen chloride in diethyl ether (40 ml, 120 mmol) was added, together with a little diethyl ether and a solid was precipitated. Collection of the solid by suction filtration followed by drying in vacuo yielded ethyl 3-methoxy-4-(1-methylpiperidin-4-ylmethoxy)benzoate (30.6 g, 100% yield) as a white solid: 1H-NMR (DMSO-d6): 7.60 (d, 1H), 7.48 (s, 1H), 7.10 (d, 1H), 4.30 (q, 2H), 3.90-4.05 (s, 2H), 3.85 (s, 3H), 3.35-3.50 (s, 2H), 2.90-3.10 (m, 2H), 2.72 (s, 3H), 2.00-2.15 (s, 1H), 1.95 (d, 2H), 1.50-1.70 (m, 2H), 1.29 (t, 3H): MS (+ve ESI): 308 (M+H)+.
References:
US7235559,2007,B1

835633-50-6
30 suppliers
inquiry

264208-60-8
11 suppliers
inquiry
