
(R)-N-1-BOC-4-CBZ-2-PIPERAZINECARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:(R)-N-1-BOC-4-CBZ-2-PIPERAZINECARBOXYLIC ACID METHYL ESTER
- CAS Number:278790-00-4
- Molecular formula:C19H26N2O6
- Molecular Weight:378.42

74-88-4
341 suppliers
$15.00/10g
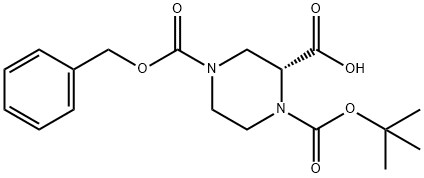
138775-02-7
71 suppliers
$56.00/250mg

278790-00-4
20 suppliers
$299.51/1g
Yield:278790-00-4 63%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20; for 3 h;
Steps:
1
PREPARATIVE EXAMPLE 1; (2R) -1-[(tert-butyl)oxycarbonyl]-4-[benzyloxycarbonyl]piperazine-2-carboxylic acid (see Preparative Example 49 in U.S. 6,372,747) (2.9 gm, 7.96 mmol) was stirred in DMF (50 ml_), methyl iodide (1.5 ml_, 23.81 mmol), and cesium carbonate (7.78 gm, 23.87 mmol) at room temperature for three hours and concentrated in vacuo. The residue was diluted with water and extracted with methylene chloride. The organics were dried over MgSO4, filtered and concentrated under reduced pressure. The crude product was purified by flash chromatography using 20% EtOAc in hexane solution as eluant to give a white foam (1.9 gm, 63% yield), FABMS: MH+ = 379.
References:
WO2006/65828,2006,A2 Location in patent:Page/Page column 90
126330-92-5
5 suppliers
inquiry

278790-00-4
20 suppliers
$299.51/1g
126330-91-4
18 suppliers
$194.93/250mg

278790-00-4
20 suppliers
$299.51/1g

24424-99-5
821 suppliers
$13.50/25G

405175-79-3
46 suppliers
$272.00/250mg

278790-00-4
20 suppliers
$299.51/1g