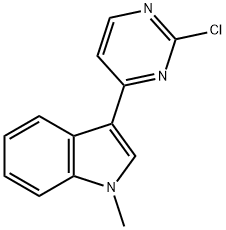
3-(2-chloropyriMidin-4-yl)-1-Methylindole synthesis
- Product Name:3-(2-chloropyriMidin-4-yl)-1-Methylindole
- CAS Number:1032452-86-0
- Molecular formula:C13H10ClN3
- Molecular Weight:243.69
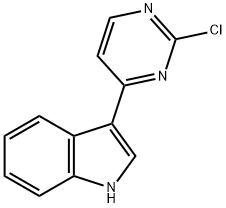
945016-63-7
80 suppliers
$44.00/100mg

74-88-4
340 suppliers
$15.00/10g

1032452-86-0
287 suppliers
$9.00/1g
Yield:1032452-86-0 96%
Reaction Conditions:
Stage #1: 3-(2-chloro-pyrimidin-4-yl)-1H-indolewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran;mineral oil at 0; for 3 h;
Steps:
130 Intermediate 130: 3-(2-Chloropyrimidin-4-yl)-1-methylindole
NaH (1.707 g, 42.68 mmol, 40% dispersion in mineral oil) was added in small portions to a cooled (0°C) mixture of 3-(2-chloropyrimidin-4-yl)-1H-indole (Intermediate 131, 8.168 g, 35.57 mmol) in THF (250 mL). The resulting mixture was stirred at 0°C for 0.5h and then CH3I (2.67 mL, 42.68 mmol) was added and the mixture stirred at 0°C for a further 3h. The reaction was quenched by the addition of sat. NaHCO3 (25 mL). The mixture was then diluted with EtOAc (100 mL), and the resulting solution was washed with sat. NaHCO3 (50 mL), water (50 mL) and sat. brine (50 mL). The organic solution was then concentrated in vacuo. Purification by FCC, eluting with 0-20% CH3OH in CH2Cl2 gave the title compound (8.35 g, 96%) as a pale yellow solid; 1H NMR: 3.90 (3H, s), 7.30 (2H, pd), 7.54-7.60 (1H, m), 7.82 (1H, d), 8.38-8.44 (1H, m), 8.49 (1H, s), 8.53 (1H, d); m/z: ES+ MH+ 244.
References:
WO2013/14448,2013,A1 Location in patent:Page/Page column 138
3934-20-1
709 suppliers
$9.00/1g
603-76-9
413 suppliers
$18.00/5g

1032452-86-0
287 suppliers
$9.00/1g

945016-63-7
80 suppliers
$44.00/100mg

50-00-0
846 suppliers
$10.00/25g

1032452-86-0
287 suppliers
$9.00/1g
120-72-9
621 suppliers
$6.00/25g

3934-20-1
709 suppliers
$9.00/1g

75-16-1
264 suppliers
$12.00/10ml

1032452-86-0
287 suppliers
$9.00/1g

120-72-9
621 suppliers
$6.00/25g

1032452-86-0
287 suppliers
$9.00/1g