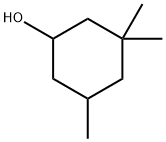
3,3,5-Trimethylcyclohexanol synthesis
- Product Name:3,3,5-Trimethylcyclohexanol
- CAS Number:116-02-9
- Molecular formula:C9H18O
- Molecular Weight:142.24
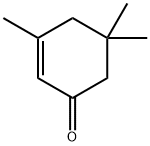
78-59-1
585 suppliers
$11.00/25g
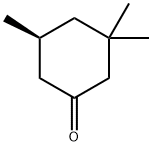
33496-83-2
0 suppliers
inquiry

116-02-9
230 suppliers
$8.00/5g
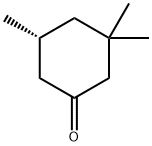
33496-82-1
3 suppliers
inquiry
Yield: 84 % ee
Reaction Conditions:
with (acetylacetonato)dicarbonylrhodium (l);(1S,1′S)-(+)-(9,9-dimethyl-9H-xanthene-4,5-diyl)bis((2-methoxyphenyl)(phenyl)phosphine);hydrogen in toluene at 40; under 37503.8 Torr; for 8 h;Autoclave;enantioselective reaction;Solvent;Reagent/catalyst;
Steps:
4.2. General procedure for the asymmetric hydrogenation
General procedure: All reactions were carried out using an automatic device[HPChemScan, Fa. HEL Ltd.] which allows the parallel hydrogenation in up to eight mini-autoclaves (16 mL). Every autoclave was equipped with a proper glass vial and loaded with 0.5 mmmol Rh precursor and 0.6 mmol of ligand 2 or 6. After assembling of autoclavesinto the device isophorone (1 mmol, 3 mL of a 0.333 M stocksolution) was added under argon atmosphere. The autoclaves were purged three times with argon (0.6 MPa) and then three times with hydrogen (1 MPa). The autoclaves were heated to 40 C under ambient pressure. Then hydrogen pressure was adjusted to 5 MPa.When the reaction was finished the system was cooled down and after release of pressure the autoclaves were purged again with argon (5 cycles). The conversion and parts of product and undesire dalcohol were estimated by NMR. The enantioselectivities were determined by gas chromatography.
References:
Börner, Armin;Gandelman, Mark;Holz, Jens;Spannenberg, Anke;Wenzel, Gudrun [Tetrahedron,2020]

78-59-1
585 suppliers
$11.00/25g

116-02-9
230 suppliers
$8.00/5g
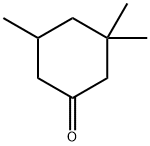
873-94-9
143 suppliers
$9.00/1g

116-02-9
230 suppliers
$8.00/5g