
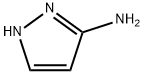
3-Acetylaminopyrazole synthesis
- Product Name:3-Acetylaminopyrazole
- CAS Number:3553-12-6
- Molecular formula:C5H7N3O
- Molecular Weight:125.13
1820-80-0
485 suppliers
$5.00/5g

108-24-7
5 suppliers
$14.00/250ML

3553-12-6
67 suppliers
$120.00/1 g
Yield:3553-12-6 65%
Reaction Conditions:
with sodium hydrogencarbonate in waterReflux;
Steps:
1
Intermediate 1: N-1H-pyrazol-3-ylacetamide 1H-pyrazol-3-amine (ALDRICH, 11.32 g, 0.136 mol) was dissolved in 100 mL of distilled water. NaHCO3 (34 g, 0.408 mol) was slowly added. Acetic anhydride (27.55 g, 0.272 mol) was then added dropwise and the resulting suspension was heated at reflux overnight. Then, the mixture was allowed to cool down to r.t. and the solid obtained was filtered off and characterized as the title compound (8.4 g, 0.067 mol, 49%). After concentration of the filtrate, a second precipitate was obtained (2.7 g, 0.021 mol, 16%), also characterized as the title compound. 1H NMR (300 MHz, DMSO-d6) δ ppm: 12.23 (br s, 1H), 10.30 (br s, 1H), 7.55 (br s, 1H), 6.45 (br s, 1H), 1.97 (s, 3H). [ES+MS] m/z 126 (MH+).
References:
Ballell Pages, Lluis;Castro Pichel, Julia;Fernandez Menendez, Raquel;Fernandez Velando, Esther Pilar;Gonzalez Del Valle, Silvia;Leon Diaz, Maria Luisa;Mendoza Losana, Alfonso;Wolfendale, Matthew James US2012/95064, 2012, A1 Location in patent:Page/Page column 15
72159-21-8
4 suppliers
inquiry

3553-12-6
67 suppliers
$120.00/1 g