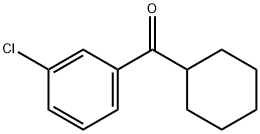
3-CHLOROPHENYL CYCLOHEXYL KETONE synthesis
- Product Name:3-CHLOROPHENYL CYCLOHEXYL KETONE
- CAS Number:211985-77-2
- Molecular formula:C13H15ClO
- Molecular Weight:222.71
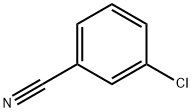
766-84-7
266 suppliers
$9.00/10g
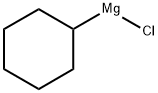
931-51-1
130 suppliers
$45.25/100ml

211985-77-2
17 suppliers
$90.00/50mg
Yield:211985-77-2 97%
Reaction Conditions:
Stage #1: 3-chloro-benzonitrile;cyclohexylmagnesiumchloride;copper(l) chloride in tetrahydrofuran;diethyl ether; for 0.5 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;diethyl ether;water at 20;
Stage #3: with water;hydrogenchloride at 90; for 1 h;
Steps:
5E.a
Preparation of N-(4-chloro-2-cyclohexanecarbonylphenyl)trifluoromethanesulfonamide (Formula 29E). a) To a stirred solution of 3-chlorobenzonitrile (2.0 g, 14.54 mmol) and cyclohexylmagnesium chloride (2M in Et2O) (8.0 mL, 15.99 mmol) in THF (20 mL) was added CuCl (29 mg, 0.29 mmol), and the mixture was refluxed for 30 minutes. After cooling to RT, cold 1N HCl (10 mL) was added cautiously, the THF removed under reduced pressure, further 1N HCl (30 mL) added and the reaction heated at 90° C. for 1 hour. To the cooled reaction mixture was added water (20 mL) and CH2Cl2 (50 mL), the phases separated, and the aqueous phase again extracted with CH2Cl2. The combined organics were washed with water, dried over MgSO4 and the solvent evaporated under vacuum. The residue was filtered through a pad of silica (eluting with CH2Cl2) to afford (3-chlorophenyl)cyclohexylmethanone 26E (3.14 g, 97%), as a yellow liquid.
References:
US2006/63841,2006,A1 Location in patent:Page/Page column 34; 8/12
110-82-7
750 suppliers
$10.00/25g
587-04-2
421 suppliers
$7.00/25g

211985-77-2
17 suppliers
$90.00/50mg

110-82-7
750 suppliers
$10.00/25g
201230-82-2
1 suppliers
inquiry

4405-42-9
18 suppliers
$400.00/1g

211985-77-2
17 suppliers
$90.00/50mg

931-51-1
130 suppliers
$45.25/100ml

211985-77-2
17 suppliers
$90.00/50mg
618-46-2
353 suppliers
$9.00/25g

211985-77-2
17 suppliers
$90.00/50mg