
3-Iodo-3H-pyrazolo[3,4-d]pyriMidin-4-aMine synthesis
- Product Name:3-Iodo-3H-pyrazolo[3,4-d]pyriMidin-4-aMine
- CAS Number:570409-85-7
- Molecular formula:C5H4IN5
- Molecular Weight:261.0232
![3-Iodo-3H-pyrazolo[3,4-d]pyriMidin-4-aMine](/CAS/20150408/GIF/570409-85-7.gif)
570409-85-7
22 suppliers
inquiry
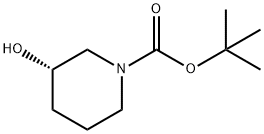
143900-44-1
555 suppliers
$5.00/1g
![(3R)-1-Boc-3-(4-aMino-3-iodo-1H-pyrazolo[3,4-d]pyriMidin-1-yl)piperidine](/CAS/20150408/GIF/1276110-38-3.gif)
1276110-38-3
51 suppliers
$18.00/100mg
Yield:1276110-38-3 33%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 10 - 20; for 12.5 h;
Steps:
9.1
Step.A mixture of 3~iodo- 3H-pyrazoio[3,4-d]pyrimidin-4-amme ( 5,9g. 22,6 mmol, 3.00 equiv), (S)-tert-butyB-hydroxypiperidine- l-carboxylaie (10g,50mmol, 2.2equiv), triphenyiphosphine ( 1 1.8 g, 45 mmol, 2 equiv) in tetrahydrofuran (300 mL) was stirred at 10 °C. Diisopropyl azodicarboxylate in tetrahydrofuran (30 mL) was dropped in die mixture slowly in 30 min.The resulting mixture was stirred for 12 h at room temperature was and then concentrated under vacuum. The residue was loaded onto a silica gel column and eluted with dichloromethane/methanol (100/1) to give 3 g (33%) of (R)-tert-butyl 3-(4-am'mo-3-iodo- 1 H- pyrazolo[3,4~dJpyrimidin-l-yl)piperidine-l -carboxyIate as yellow solid. MS (ESI, pos. ion) m/z: 45 (M+l).
References:
WO2012/158764,2012,A1 Location in patent:Page/Page column 172