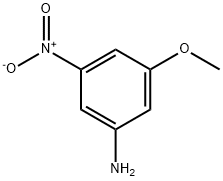
3-METHOXY-5-NITROANILINE synthesis
- Product Name:3-METHOXY-5-NITROANILINE
- CAS Number:586-10-7
- Molecular formula:C7H8N2O3
- Molecular Weight:168.15
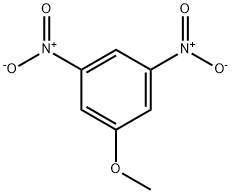
5327-44-6
39 suppliers
$75.00/2 g

586-10-7
58 suppliers
inquiry
Yield: 93%
Reaction Conditions:
with sodium sulfide;sodium hydrogencarbonate in methanol;water for 0.5 h;Heating / reflux;
Steps:
12.1
Example 124-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-thiophen-3-yl-phenoxy)-hexyl]-2-(2-carboxy-ethyl)-phenoxy}-butyric acid Step 1: Preparation of 3-methoxy-5-nitro-phenylamine; Sodium bicarbonate (5.62 g, 66.87 mmol) was added to a solution of sodium sulfide (5.5 g, 70.58 mmol) in deionized water (60 mL). When the sodium bicarbonate was completely dissolved, methanol (50 mL) was added, and the solution was cooled to 0° C. A precipitate formed, which was removed by filtration through a Celite pad. The filtered solution was added quickly to a solution of 3,5-dinitroanisole (7.36 g, 37.15 mmol) in methanol (50 mL). The resulting suspension was heated to reflux for 30 min and then the solution was concentrated in vacuo to remove methanol. The aqueous residue was poured into 200 mL of ice-water, and the resulting orange precipitate was collected by filtration. After air-drying, 3-methoxy-5-nitro-phenylamine (5.82 g, 93%) was obtained as light brown solid: ES(+)-HRMS m/e calculated for C7H8N2O3 (M+H)+ 169.0608, found 169.0608.
References:
Dominique, Romyr;Fotouhi, Nader;Gillespie, Paul;Goodnow, Robert Alan;Kowalczyk, Agnieszka;Qiao, Qi;Sidduri, Achyutharao US2009/54466, 2009, A1 Location in patent:Page/Page column 29

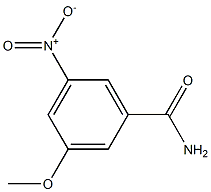
116496-83-4
0 suppliers
inquiry

586-10-7
58 suppliers
inquiry
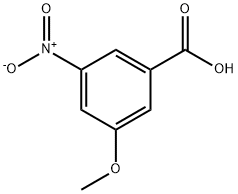
292635-40-6
7 suppliers
inquiry

586-10-7
58 suppliers
inquiry
78238-12-7
118 suppliers
$8.00/100mg

586-10-7
58 suppliers
inquiry
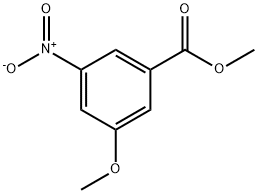
78238-13-8
54 suppliers
$90.00/100mg

586-10-7
58 suppliers
inquiry