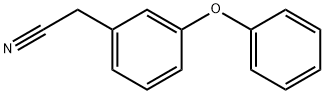
3-PHENOXYPHENYLACETONITRILE synthesis
- Product Name:3-PHENOXYPHENYLACETONITRILE
- CAS Number:51632-29-2
- Molecular formula:C14H11NO
- Molecular Weight:209.24
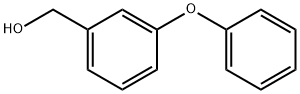
13826-35-2
325 suppliers
$6.00/5g
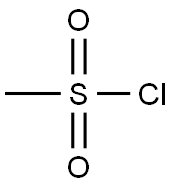
124-63-0
6 suppliers
$10.00/5g

51632-29-2
93 suppliers
$47.00/500 mg
Yield: 32%
Reaction Conditions:
with sodium iodide;triethylamine in dimethyl sulfoxide;ethyl acetate;acetone
Steps:
R.28 Reference Example 28
Reference Example 28 To a solution of 3-phenoxybenzylalcohol (25.0 g) and triethylamine (26.3 ml) in ethyl acetate (300 ml), methanesulfonyl chloride (14.6 ml) was added dropwise at 0° C. After stirring for 1 hour, the reaction mixture was washed with saturated aqueous sodium chloride, dried (MgSO4) and concentrated. The residue was dissolved in acetone (300 ml), admixed with sodium iodide (37.5 g) and then stirred for 1 hour. The reaction mixture was concentrated, and the residue was diluted with water and then extracted with ethyl acetate. The ethyl acetate layer was washed with water, dried (MgSO4), and then concentrated. The residue was dissolved in dimethyl sulfoxide (100 ml) and stirred with sodium cyanide (7.35 g) for 15 hours at room temperature. The reaction mixture was diluted with ethyl acetate, washed with water, dried (MgSO4), and then concentrated. The residue was subjected to column chromatography on silica gel to obtain 3-phenoxyphenylacetonitrile (8.36 g, yield 32%) as an oil from a fraction eluted with ethyl acetate-hexane (1:7. v/v). NMR(CDCl3) δ: 3.72(2H, s), 6.90-7.20(6H, m), 7.28-7.43(3H, m).
References:
Takeda Chemical Industries, Ltd. US6251926, 2001, B1
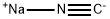
773837-37-9
0 suppliers
inquiry
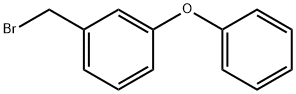
51632-16-7
96 suppliers
$74.23/250mgs:

51632-29-2
93 suppliers
$47.00/500 mg
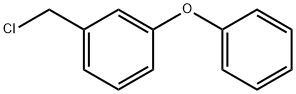
53874-66-1
149 suppliers
$14.00/1g
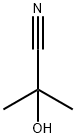
75-86-5
2 suppliers
$80.00/5g

51632-29-2
93 suppliers
$47.00/500 mg
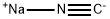
773837-37-9
0 suppliers
inquiry

53874-66-1
149 suppliers
$14.00/1g

51632-29-2
93 suppliers
$47.00/500 mg

51632-16-7
96 suppliers
$74.23/250mgs:
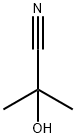
75-86-5
2 suppliers
$80.00/5g

51632-29-2
93 suppliers
$47.00/500 mg