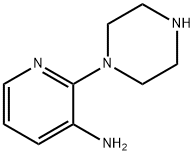
3-Pyridinamine,2-(1-piperazinyl)-(9CI) synthesis
- Product Name:3-Pyridinamine,2-(1-piperazinyl)-(9CI)
- CAS Number:87394-62-5
- Molecular formula:C9H14N4
- Molecular Weight:178.23
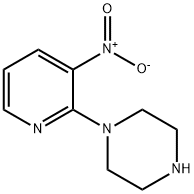
87394-48-7
68 suppliers
$14.00/100mg

87394-62-5
12 suppliers
$257.00/1g
Yield:87394-62-5 72%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethanol at 25; under 2068.65 Torr; for 12 h;
Steps:
I.1 Intermediate compound 75:
To a solution of intermediate compound 74 (500 mg, 2.40 mmol, 1 eq) in EtOH (20 mL) was added wet Pd/C (511.10 mg, 240.13 umol, 5% purity, 0.1 eq) under N2. The suspension was degassed under vacuum and purged with H2 several times. The mixture was stirred under H2 (40 psi) at 25 °C for 12 hr. The reaction mixture was filtered. The filter cake was washed with MeOH (50 mL x 3). The filtrate was concentrated and the residue was purified by prep-HPLC (column: Phenomenex luna Cl 8 150*25mm* 10um;mobile phase: [water(0.225%FA)-ACN];B%: 13%-43%,10min) to give the intermediate compound 75 (390 mg, 72 % yield) as a white solid.LCMS (ESI position ion) m/z: (M+H)+: 179.1 (calculated: 178.12)1H NMR (400 MHz, CHLOROFORM-d) d ppm 8.51 (s, 1H), 7.84 - 7.76 (m, 1H), 7.03 - 6.95 (m, 1H), 6.93 - 6.84 (m, 1H), 3.49 - 3.25 (m, 8H).
References:
WO2021/170797,2021,A1 Location in patent:Page/Page column 134; 174
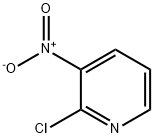
5470-18-8
435 suppliers
$6.00/5g

87394-62-5
12 suppliers
$257.00/1g