
4-[2-(Dimethylamino)-1-(1-hydroxycyclohexyl)ethyl]-phenol hydrochloride synthesis
- Product Name:4-[2-(Dimethylamino)-1-(1-hydroxycyclohexyl)ethyl]-phenol hydrochloride
- CAS Number:300827-87-6
- Molecular formula:C16H25NO2HCl
- Molecular Weight:0

99300-78-4
634 suppliers
$5.00/25mg
![4-[2-(Dimethylamino)-1-(1-hydroxycyclohexyl)ethyl]-phenol hydrochloride](/CAS/GIF/300827-87-6.gif)
300827-87-6
28 suppliers
$227.00/10mg
Yield:300827-87-6 88.5%
Reaction Conditions:
with methanesulfonic acid;L-methionineLarge scale;
Steps:
1-3 Example 2 A method for preparing desvenlafaxine hydrochloride
Dissolve venlafaxine hydrochloride in methanesulfonic acid and demethylate it to methionine; the molar ratio of the amount of methionine added to venlafaxine hydrochloride is 1.2: 1. The reaction chemical formula is as follows: Formula III is venlafaxine hydrochloride; Formula IV is desvenlafaxine hydrochloride.After the above reaction is completed, the purification steps are as follows:(1) Stir the reaction body at 75-80 ° C for 16h. After cooling to 20 ° C, slowly add water. The volume ratio of added water to the reaction system is 1.3: 2. Use sodium hydroxide with a mass fraction of 32% Adjust the pH to 11, maintain the temperature at 38 ° C, add ethyl acetate, stir at 150r / m for 15min, separate the organic phase and the inorganic phase, the volume ratio of ethyl acetate added to the reaction system is 1.5: 2. Add activated carbon to the organic phase and stir for 30 minutes, then filter, the diameter of the filter mesh is 10-50 microns, and the filtrate is distilled under reduced pressure at 45 and a pressure of 50 mb to obtain the crude product;(2) Dissolve the crude product in acetone at 20-25 ° C while stirring to introduce hydrogen chloride gas for reaction. The molar ratio of the amount of hydrogen chloride gas to venlafaxine hydrochloride is 1.1-1.2: 1. After adding hydrogen chloride gas, the suspension was stirred at 4 ° C for 3h and then filtered. The diameter of the filter mesh was 10-50 microns. The filter cake and isopropanol were added to the reaction vessel and heated to reflux. After 15min, the system was cooled down To 0-5 , stirred for 3h, dried under reduced pressure at 35 , 150mb, to get desvenlafaxine hydrochloride, the yield is 88.5%, the purity is 99.9%.
References:
CN104628582,2016,B Location in patent:Paragraph 0015-0032
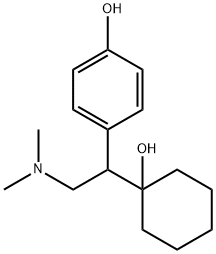
93413-62-8
241 suppliers
$5.00/5mg
![4-[2-(Dimethylamino)-1-(1-hydroxycyclohexyl)ethyl]-phenol hydrochloride](/CAS/GIF/300827-87-6.gif)
300827-87-6
28 suppliers
$227.00/10mg
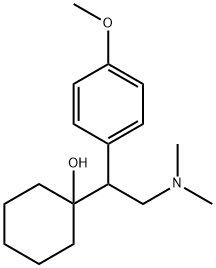
93413-69-5
255 suppliers
$10.00/1mg
![4-[2-(Dimethylamino)-1-(1-hydroxycyclohexyl)ethyl]-phenol hydrochloride](/CAS/GIF/300827-87-6.gif)
300827-87-6
28 suppliers
$227.00/10mg